MicroRNAs: regulators of neuronal fate
- PMID: 23374323
- PMCID: PMC3836262
- DOI: 10.1016/j.ceb.2012.12.007
MicroRNAs: regulators of neuronal fate
Abstract
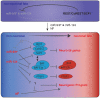
Mammalian neural development has been traditionally studied in the context of evolutionarily conserved signaling pathways and neurogenic transcription factors. Recent studies suggest that microRNAs, a group of highly conserved noncoding regulatory small RNAs also play essential roles in neural development and neuronal function. A part of their action in the developing nervous system is to regulate subunit compositions of BAF complexes (ATP-dependent chromatin remodeling complexes), which appear to have dedicated functions during neural development. Intriguingly, ectopic expression of a set of brain-enriched microRNAs, miR-9/9* and miR-124 that promote the assembly of neuron-specific BAF complexes, converts the nonneuronal fate of human dermal fibroblasts towards postmitotic neurons, thereby revealing a previously unappreciated instructive role of these microRNAs. In addition to these global effects, accumulating evidence indicates that many microRNAs could also function locally, such as at the growth cone or at synapses modulating synaptic activity and neuronal connectivity. Here we discuss some of the recent findings about microRNAs' activity in regulating various developmental stages of neurons.
Copyright © 2013. Published by Elsevier Ltd.
Figures

References
-
- Pasquinelli A. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature reviews. Genetics. 13:271–282. - PubMed
-
- Tay Y, Zhang J, Thomson A, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. - PubMed
-
-
Yoo A, Staahl B, Chen L, Crabtree G. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. ** This paper described a series of elegantly designed BAC-transgenic reporter mouse embryos that demonstrated BAF53a to be simultaneously targeted by both miR-124 and miR-9*. Further experiments delineated a negative genetic circuit centered around these miRNAs that regulates neural progenitor mitotic exit and dendritic morphogenesis.
-
-
- Peter ME. Targeting of mRNAs by multiple miRNAs: the next step. Oncogene. 29:2161–2164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

