Conformational coupling across the plasma membrane in activation of the EGF receptor
- PMID: 23374349
- PMCID: PMC3718647
- DOI: 10.1016/j.cell.2012.12.032
Conformational coupling across the plasma membrane in activation of the EGF receptor
Abstract
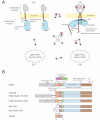
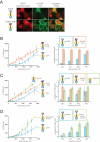
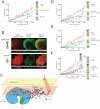
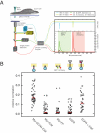
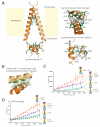
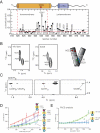
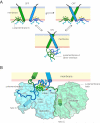
How the epidermal growth factor receptor (EGFR) activates is incompletely understood. The intracellular portion of the receptor is intrinsically active in solution, and to study its regulation, we measured autophosphorylation as a function of EGFR surface density in cells. Without EGF, intact EGFR escapes inhibition only at high surface densities. Although the transmembrane helix and the intracellular module together suffice for constitutive activity even at low densities, the intracellular module is inactivated when tethered on its own to the plasma membrane, and fluorescence cross-correlation shows that it fails to dimerize. NMR and functional data indicate that activation requires an N-terminal interaction between the transmembrane helices, which promotes an antiparallel interaction between juxtamembrane segments and release of inhibition by the membrane. We conclude that EGF binding removes steric constraints in the extracellular module, promoting activation through N-terminal association of the transmembrane helices.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Becker W, Bergmann A, Geddes CD, Lakowicz JR. Multi-Dimensional Time-Correlated Single Photon Counting. Vol 2005. Springer; US: 2005.
-
- Bocharov EV, Mineev KS, Volynsky PE, Ermolyuk YS, Tkach EN, Sobol AG, Chupin VV, Kirpichnikov MP, Efremov RG, Arseniev AS. Spatial structure of the dimeric transmembrane domain of the growth factor receptor ErbB2 presumably corresponding to the receptor active state. J Biol Chem. 2008;283:6950–6956. - PubMed
-
- Breeze AL. Isotope-filtered NMR methods for the study of biomolecular structure and interactions. Prog Nucl Mag Res Sp. 2000;36:323–372.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

