MST1, a key player, in enhancing fast skeletal muscle atrophy
- PMID: 23374633
- PMCID: PMC3606410
- DOI: 10.1186/1741-7007-11-12
MST1, a key player, in enhancing fast skeletal muscle atrophy
Abstract
Background: Skeletal muscle undergoes rapid atrophy upon denervation and the underlying mechanisms are complicated. FOXO3a has been implicated as a major mediator of muscle atrophy, but how its subcellular location and activity is controlled during the pathogenesis of muscle atrophy remains largely unknown. MST1 (Mammalian Sterile 20-like kinase 1) is identified as a central component of the Hippo signaling pathway. MST1 has been shown to mediate phosphorylation of FOXO3a at Ser207. Whether this MST1-FOXO signaling cascade exerts any functional consequence on cellular homeostasis remains to be investigated.
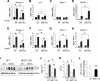
Result: We identified that MST1 kinase was expressed widely in skeletal muscles and was dramatically up-regulated in fast- but not slow-dominant skeletal muscles immediately following denervation. The results of our histological and biochemical studies demonstrated that deletion of MST1 significantly attenuated denervation-induced skeletal muscle wasting and decreased expression of Atrogin-1 and LC3 genes in fast-dominant skeletal muscles from three- to five-month-old adult mice. Further studies indicated that MST1, but not MST2, remarkably increased FOXO3a phosphorylation level at Ser207 and promoted its nuclear translocation in atrophic fast-dominant muscles.
Conclusions: We have established that MST1 kinase plays an important role in regulating denervation-induced skeletal muscle atrophy. During the early stage of muscle atrophy, the up-regulated MST1 kinase promoted progression of neurogenic atrophy in fast-dominant skeletal muscles through activation of FOXO3a transcription factors.
Figures



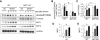
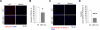

Similar articles
-
Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing's syndrome.Am J Physiol Endocrinol Metab. 2017 Jun 1;312(6):E495-E507. doi: 10.1152/ajpendo.00389.2016. Epub 2017 Feb 28. Am J Physiol Endocrinol Metab. 2017. PMID: 28246104
-
AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1.J Cell Biochem. 2012 Feb;113(2):695-710. doi: 10.1002/jcb.23399. J Cell Biochem. 2012. PMID: 22006269
-
Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway.J Trace Elem Med Biol. 2016 May;35:66-76. doi: 10.1016/j.jtemb.2016.01.011. Epub 2016 Jan 28. J Trace Elem Med Biol. 2016. PMID: 27049128
-
Molecular basis for statin-induced muscle toxicity: implications and possibilities.Pharmacogenomics. 2008 Aug;9(8):1133-42. doi: 10.2217/14622416.9.8.1133. Pharmacogenomics. 2008. PMID: 18681786 Review.
-
Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis.Nat Rev Mol Cell Biol. 2011 Jun;12(6):349-61. doi: 10.1038/nrm3118. Nat Rev Mol Cell Biol. 2011. PMID: 21602905 Review.
Cited by
-
FOXO transcription factors: key regulators of cellular quality control.Trends Biochem Sci. 2014 Apr;39(4):159-69. doi: 10.1016/j.tibs.2014.02.003. Epub 2014 Mar 13. Trends Biochem Sci. 2014. PMID: 24630600 Free PMC article. Review.
-
Arrhythmogenic Cardiomyopathy and Skeletal Muscle Dystrophies: Shared Histopathological Features and Pathogenic Mechanisms.Front Physiol. 2020 Jul 30;11:834. doi: 10.3389/fphys.2020.00834. eCollection 2020. Front Physiol. 2020. PMID: 32848821 Free PMC article. Review.
-
MicroRNA 322 Aggravates Dexamethasone-Induced Muscle Atrophy by Targeting IGF1R and INSR.Int J Mol Sci. 2020 Feb 7;21(3):1111. doi: 10.3390/ijms21031111. Int J Mol Sci. 2020. PMID: 32046161 Free PMC article.
-
The Hippo Signaling Pathway in the Regulation of Skeletal Muscle Mass and Function.Exerc Sport Sci Rev. 2018 Apr;46(2):92-96. doi: 10.1249/JES.0000000000000142. Exerc Sport Sci Rev. 2018. PMID: 29346163 Free PMC article. Review.
-
Forkhead box O1 and muscle RING finger 1 protein expression in atrophic and hypertrophic denervated mouse skeletal muscle.J Mol Signal. 2014 Sep 24;9:9. doi: 10.1186/1750-2187-9-9. eCollection 2014. J Mol Signal. 2014. PMID: 25276226 Free PMC article.
References
-
- Carvalho RF, Castan EP, Coelho CA, Lopes FS, Almeida FL, Michelin A, de Souza RW, Araujo JP Jr, Cicogna AC, Dal Pai-Silva M. Heart failure increases atrogin-1 and MuRF1 gene expression in skeletal muscle with fiber type-specific atrophy. J Mol Histol. 2010;41:81–87. doi: 10.1007/s10735-010-9262-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

