Effects of early life exposure to ultraviolet C radiation on mitochondrial DNA content, transcription, ATP production, and oxygen consumption in developing Caenorhabditis elegans
- PMID: 23374645
- PMCID: PMC3621653
- DOI: 10.1186/2050-6511-14-9
Effects of early life exposure to ultraviolet C radiation on mitochondrial DNA content, transcription, ATP production, and oxygen consumption in developing Caenorhabditis elegans
Abstract
Background: Mitochondrial DNA (mtDNA) is present in multiple copies per cell and undergoes dramatic amplification during development. The impacts of mtDNA damage incurred early in development are not well understood, especially in the case of types of mtDNA damage that are irreparable, such as ultraviolet C radiation (UVC)-induced photodimers.
Methods: We exposed first larval stage nematodes to UVC using a protocol that results in accumulated mtDNA damage but permits nuclear DNA (nDNA) repair. We then measured the transcriptional response, as well as oxygen consumption, ATP levels, and mtDNA copy number through adulthood.
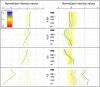
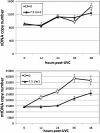
Results: Although the mtDNA damage persisted to the fourth larval stage, we observed only a relatively minor ~40% decrease in mtDNA copy number. Transcriptomic analysis suggested an inhibition of aerobic metabolism and developmental processes; mRNA levels for mtDNA-encoded genes were reduced ~50% at 3 hours post-treatment, but recovered and, in some cases, were upregulated at 24 and 48 hours post-exposure. The mtDNA polymerase γ was also induced ~8-fold at 48 hours post-exposure. Moreover, ATP levels and oxygen consumption were reduced in response to UVC exposure, with marked reductions of ~50% at the later larval stages.
Conclusions: These results support the hypothesis that early life exposure to mitochondrial genotoxicants could result in mitochondrial dysfunction at later stages of life, thereby highlighting the potential health hazards of time-delayed effects of these genotoxicants in the environment.
Figures

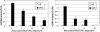


References
-
- Schmidt CW. Mito-conundrum unraveling environmental effects on mitochondria. Environ Heal Perspect. 2010;118:A292–A297. doi: 10.1289/ehp.118-a292. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

