Communication training for advanced medical students improves information recall of medical laypersons in simulated informed consent talks--a randomized controlled trial
- PMID: 23374907
- PMCID: PMC3598682
- DOI: 10.1186/1472-6920-13-15
Communication training for advanced medical students improves information recall of medical laypersons in simulated informed consent talks--a randomized controlled trial
Abstract
Background: Informed consent talks are mandatory before invasive interventions. However, the patients' information recall has been shown to be rather poor. We investigated, whether medical laypersons recalled more information items from a simulated informed consent talk after advanced medical students participated in a communication training aiming to reduce a layperson's cognitive load.
Methods: Using a randomized, controlled, prospective cross-over-design, 30 5th and 6th year medical students were randomized into two groups. One group received communication training, followed by a comparison intervention (early intervention group, EI); the other group first received the comparison intervention and then communication training (late intervention group, LI). Before and after the interventions, the 30 medical students performed simulated informed consent talks with 30 blinded medical laypersons using a standardized set of information. We then recorded the number of information items the medical laypersons recalled.
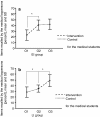
Results: After the communication training both groups of medical laypersons recalled significantly more information items (EI: 41 ± 9% vs. 23 ± 9%, p < .0001, LI 49 ± 10% vs. 35 ± 6%, p < .0001). After the comparison intervention the improvement was modest and significant only in the LI (EI: 42 ± 9% vs. 40 ± 9%, p = .41, LI 35 ± 6% vs. 29 ± 9%, p = .016).
Conclusion: Short communication training for advanced medical students improves information recall of medical laypersons in simulated informed consent talks.
Figures

References
-
- Meisel A, Roth LH, Lidz CW. Toward a model of the legal doctrine of informed consent. Am J Psychiatry. 1977;134(3):285–289. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

