Eukaryotic virulence determinants utilize phosphoinositides at the ER and host cell surface
- PMID: 23375057
- PMCID: PMC3595378
- DOI: 10.1016/j.tim.2012.12.004
Eukaryotic virulence determinants utilize phosphoinositides at the ER and host cell surface
Abstract
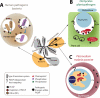
Similar to bacteria, eukaryotic pathogens may utilize common strategies of pathogenic secretion, because effector proteins from the oomycete Phytophthora infestans and virulence determinants from the human malaria parasite Plasmodium falciparum share a functionally equivalent host-cell-targeting motif (RxLR-dEER in P. infestans and RxLxE/D/Q in P. falciparum). Here we summarize recent studies that reveal that the malarial motif may function differently than previously envisioned. Binding of the lipid phosphatidylinositol 3-phosphate [PI(3)P] is a critical step in accessing the host for both pathogens, but occurs in different locations. Nanomolar affinity for PI(3)P by these short amino acid motifs suggests that a newly identified mechanism of phosphoinositide binding that unexpectedly occurs in secretory locations has been exploited for virulence by diverse eukaryotic pathogens.
Published by Elsevier Ltd.
Figures



References
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
-
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. - PubMed
-
- Dai S, et al. Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic. 2007;8:1365–1374. - PubMed
-
- Galan JE. Salmonella interactions with host cells: Type III secretion at work. Ann. Rev. Cell Dev. Biol. 2001;17:53–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

