Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells
- PMID: 23375059
- PMCID: PMC3644521
- DOI: 10.1016/j.metabol.2013.01.003
Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells
Abstract
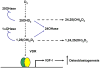
There are many human extra-renal tissues and cells that biosynthesize 1α,25-dihydroxyvitamin D (1α,25(OH)(2)D) by the action of CYP27B1/1α-hydroxylase. Human marrow stromal cells (hMSCs), also known as mesenchymal stem cells, were isolated from marrow discarded from well-characterized, consented subjects during common orthopedic procedures. Human MSCs can give rise to osteoblasts, chondrocytes, adipocytes, and other lineages. Their in vitro differentiation to osteoblasts is stimulated by 1α,25(OH)(2)D, and recent evidence indicates that they have the capacity to metabolize vitamin D in a regulated manner. Human MSCs express the vitamin D receptor, 25-hydroxylases, 1α-hydroxylase, and 24-hydroxylase; stimulation of in vitro osteoblastogenesis by 25(OH)D depends on the activity of CYP27B1/1α-hydroxylase. The finding that hMSCs are a both a producer and target of 1α,25(OH)(2)D suggests a potential autocrine/paracrine role of vitamin D metabolism in osteoblast differentiation. Expression and enzyme activity of CYP27B1/1α-hydroxylase are upregulated by substrate 25(OH)D and Parathyroid Hormone (PTH) and are downregulated by 1α,25(OH)(2)D. With subject age, there are decreases in basal osteoblast potential and in stimulation of osteoblastogenesis by 1α,25(OH)(2)D, 25(OH)D, and PTH. In vitro treatment with a combination of 25(OH)D and PTH rejuvenated osteoblastogenesis with hMSCs from elders; this was attributable to increases in CYP27B1/1α-hydroxylase and in receptor for each hormone by the reciprocal factor. Other clinical variables beside age, i.e. low serum 25(OH)D or low estimated glomerular filtration rate, are correlated with reduced osteoblastogenesis. These studies suggest that osteoblastogenesis may not be optimal unless there is sufficient serum 25(OH)D substrate for hMSCs to synthesize and respond to local 1α,25(OH)(2)D.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there is no conflict of interest associated with this manuscript.
Figures


Similar articles
-
Vitamin D metabolism and action in human marrow stromal cells: effects of chronic kidney disease.J Steroid Biochem Mol Biol. 2013 Jul;136:342-4. doi: 10.1016/j.jsbmb.2012.09.009. Epub 2012 Sep 16. J Steroid Biochem Mol Biol. 2013. PMID: 22989482 Free PMC article.
-
Age-related decline in osteoblastogenesis and 1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone.Aging Cell. 2011 Dec;10(6):962-71. doi: 10.1111/j.1474-9726.2011.00735.x. Epub 2011 Aug 24. Aging Cell. 2011. PMID: 21824271 Free PMC article.
-
Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH)D3 and parathyroid hormone in MSCs from elders.J Steroid Biochem Mol Biol. 2013 Jul;136:156-9. doi: 10.1016/j.jsbmb.2012.09.002. Epub 2012 Sep 12. J Steroid Biochem Mol Biol. 2013. PMID: 22982627 Free PMC article.
-
Chronic kidney disease and vitamin D metabolism in human bone marrow-derived MSCs.Ann N Y Acad Sci. 2017 Aug;1402(1):43-55. doi: 10.1111/nyas.13464. Ann N Y Acad Sci. 2017. PMID: 28926112 Free PMC article. Review.
-
Vitamin D and bone.J Cell Biochem. 2003 Feb 1;88(2):259-66. doi: 10.1002/jcb.10331. J Cell Biochem. 2003. PMID: 12520524 Review.
Cited by
-
Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts.Nutrients. 2023 Jan 17;15(3):480. doi: 10.3390/nu15030480. Nutrients. 2023. PMID: 36771187 Free PMC article. Review.
-
Molecular mechanism of extrinsic factors affecting anti-aging of stem cells.World J Stem Cells. 2015 Mar 26;7(2):512-20. doi: 10.4252/wjsc.v7.i2.512. World J Stem Cells. 2015. PMID: 25815136 Free PMC article. Review.
-
Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling.Biomolecules. 2022 Feb 18;12(2):323. doi: 10.3390/biom12020323. Biomolecules. 2022. PMID: 35204824 Free PMC article.
-
Vitamin D receptor gene ApaI polymorphism and breast cancer susceptibility: a meta-analysis.Tumour Biol. 2014 Jan;35(1):785-90. doi: 10.1007/s13277-013-1107-2. Epub 2013 Sep 19. Tumour Biol. 2014. Retraction in: Tumour Biol. 2017 Apr 20. doi: 10.1007/s13277-017-5487-6. PMID: 24048755 Retracted.
-
Construction of a Luciferase Reporter System to Monitor Osteogenic Differentiation of Mesenchymal Stem Cells by Using a Mammalian Artificial Chromosome Vector.Yonago Acta Med. 2015 Mar;58(1):23-9. Epub 2015 Mar 27. Yonago Acta Med. 2015. PMID: 26190894 Free PMC article.
References
-
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. - PubMed
-
- Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Luk JM, Wang PP, Lee CK, et al. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

