Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA
- PMID: 23375499
- PMCID: PMC3595360
- DOI: 10.1016/j.molcel.2012.12.021
Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA
Abstract
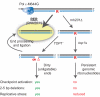
RNase H2-dependent ribonucleotide excision repair (RER) removes ribonucleotides incorporated during DNA replication. When RER is defective, ribonucleotides in the nascent leading strand of the yeast genome are associated with replication stress and genome instability. Here, we provide evidence that topoisomerase 1 (Top1) initiates an independent form of repair to remove ribonucleotides from genomic DNA. This Top1-dependent process activates the S phase checkpoint. Deleting TOP1 reverses this checkpoint activation and also relieves replication stress and genome instability in RER-defective cells. The results reveal an additional removal pathway for a very common lesion in DNA, and they imply that the "dirty" DNA ends created when Top1 incises ribonucleotides in DNA are responsible for the adverse consequences of ribonucleotides in RNase H2-defective cells.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet. 2006;38:910–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

