A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi
- PMID: 23376276
- PMCID: PMC3605662
- DOI: 10.1074/jbc.M112.433540
A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi
Abstract
The Lyme disease pathogen Borrelia burgdorferi represents a novel organism in which to study metalloprotein biology in that this spirochete has uniquely evolved with no requirement for iron. Not only is iron low, but we show here that B. burgdorferi has the capacity to accumulate remarkably high levels of manganese. This high manganese is necessary to activate the SodA superoxide dismutase (SOD) essential for virulence. Using a metalloproteomic approach, we demonstrate that a bulk of B. burgdorferi SodA directly associates with manganese, and a smaller pool of inactive enzyme accumulates as apoprotein. Other metalloproteins may have similarly adapted to using manganese as co-factor, including the BB0366 aminopeptidase. Whereas B. burgdorferi SodA has evolved in a manganese-rich, iron-poor environment, the opposite is true for Mn-SODs of organisms such as Escherichia coli and bakers' yeast. These Mn-SODs still capture manganese in an iron-rich cell, and we tested whether the same is true for Borrelia SodA. When expressed in the iron-rich mitochondria of Saccharomyces cerevisiae, B. burgdorferi SodA was inactive. Activity was only possible when cells accumulated extremely high levels of manganese that exceeded cellular iron. Moreover, there was no evidence for iron inactivation of the SOD. B. burgdorferi SodA shows strong overall homology with other members of the Mn-SOD family, but computer-assisted modeling revealed some unusual features of the hydrogen bonding network near the enzyme's active site. The unique properties of B. burgdorferi SodA may represent adaptation to expression in the manganese-rich and iron-poor environment of the spirochete.
Figures
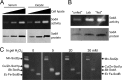
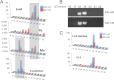






References
-
- Wintjens R., Noël C., May A. C., Gerbod D., Dufernez F., Capron M., Viscogliosi E., Rooman M. (2004) Specificity and phenetic relationships of iron- and manganese-containing superoxide dismutases on the basis of structure and sequence comparisons. J. Biol. Chem. 279, 9248–9254 - PubMed
-
- Mizuno K., Whittaker M. M., Bächinger H. P., Whittaker J. W. (2004) Calorimetric studies on the tight-binding metal interactions of Escherichia coli manganese superoxide dismutase. J. Biol. Chem. 279, 27339–27344 - PubMed
-
- Iranzo O. (2011) Manganese complexes displaying superoxide dismutase activity. A balance between different factors. Bioorg. Chem. 39, 73–87 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

