Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo
- PMID: 23376638
- PMCID: PMC3760787
- DOI: 10.1016/j.canlet.2013.01.037
Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo
Abstract
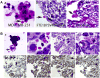
Bone marrow is generally considered the main source of erythroid cells. Here we report that a single hypoxia-mimic chemical, CoCl2, can increase the size of fibroblasts and cancer cells and lead to formation of polyploidy giant cells (PGCs) or polyploidy giant cancer cells (PGCCs), activation of stem cell marker expression, increased growth of normal and cancer spheroid, and lead to differentiation of the fibroblasts and epithelial cells toward erythroid lineage expressing hemoglobins both in vitro and in vivo. Immunohistochemical examination demonstrated that these cells are predominantly made of embryonic hemoglobins, with various levels of fetal and adult hemoglobins. Ectopic expression of c-Myc induced the generation of nucleated erythoid cells expressing variable levels of embryonic and fetal hemoglobins. Generation of these erythroid cells can be also observed via histological examination of other cancer cell lines and human tumor samples. These data suggest that normal and solid cancer cells can directly generate erythroid cells to obtain oxygen in response to hypoxia and may explain the ineffectiveness of conventional anti-angiogenic therapies for cancer, which are directed at endothelium-dependent vessels, and offer new targets for intervention.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures



References
-
- Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. - PubMed
-
- Fried W. Erythropoietin and erythropoiesis. Exp Hematol. 2009;37:1007–1015. - PubMed
-
- Tsuchida E, Komatsu T, Hamamatsu K, Matsukawa Y, Tajima A, Yoshizu A, Izumi Y, Kobayashi K. Exchange transfusion with albumin-heme as an artificialO2-infusion into anesthetized rats: physiological responses, O2-delivery, and reduction of the oxidized hemin sites by red blood cells. Bioconjug Chem. 2000;11:46–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

