Nickel inhibits β-1 adrenoceptor mediated activation of cardiac CFTR chloride channels
- PMID: 23376720
- PMCID: PMC3686155
- DOI: 10.1016/j.bbrc.2013.01.087
Nickel inhibits β-1 adrenoceptor mediated activation of cardiac CFTR chloride channels
Abstract
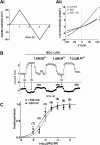
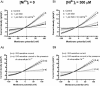
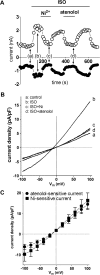
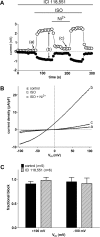
Cardiac ventricular myocytes exhibit a protein kinase A-dependent Cl(-) current (ICl.PKA) mediated by the cystic fibrosis transmembrane conductance regulator (CFTR). There is conflicting evidence regarding the ability of the divalent cation nickel (Ni(2+)), which has been used widely in vitro in the study of other cardiac ionic conductances, to inhibit ICl.PKA. Here the action of Ni(2+) on ICl.PKA activated by β-adrenergic stimulation has been elucidated. Whole-cell patch-clamp recordings were made from rabbit isolated ventricular myocytes. Externally applied Ni(2+) blocked ICl.PKA activated by 1 μM isoprenaline with a log IC50 (M) of -4.107 ± 0.075 (IC50=78.1 μM) at +100 mV and -4.322 ± 0.107 (IC50=47.6 μM) at -100 mV. Thus, the block of ICl.PKA by Ni(2+) was not strongly voltage dependent. Ni(2+) applied internally via the patch-pipette was ineffective at inhibiting isoprenaline-activated ICl,PKA, but in the same experiments the current was suppressed by external Ni(2+) application, indicative of an external site of Ni(2+) action. In the presence of 1 μM atenolol isoprenaline was ineffective at activating ICl.PKA, but in the presence of the β2-adrenoceptor inhibitor ICI 118,551 isoprenaline still activated Ni(2+)-sensitive ICl.PKA. Collectively, these data demonstrate that Ni(2+) ions produce marked inhibition of β1-adrenoceptor activated ventricular ICl.PKA at submillimolar [Ni(2+)]: an action that is likely to involve an interaction between Ni(2+) and β1-adrenoceptors. The concentration-dependence for ICl.PKA inhibition seen here indicates the potential for confounding effects on ICl,PKA to occur even at comparatively low Ni(2+) concentrations, when Ni(2+) is used to study other cardiac ionic currents under conditions of β-adrenergic agonism.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
β-Adrenoceptor/PKA-stimulation, Na(+)-Ca(2+) exchange and PKA-activated Cl(-) currents in rabbit cardiomyocytes: a conundrum.Cell Calcium. 2011 Apr;49(4):233-9. doi: 10.1016/j.ceca.2011.02.006. Epub 2011 Mar 25. Cell Calcium. 2011. PMID: 21439639 Free PMC article.
-
Cardiac ion channel current modulation by the CFTR inhibitor GlyH-101.Biochem Biophys Res Commun. 2011 Apr 29;408(1):12-7. doi: 10.1016/j.bbrc.2011.03.089. Epub 2011 Mar 31. Biochem Biophys Res Commun. 2011. PMID: 21439936
-
Effects of β-adrenoceptor stimulation on delayed rectifier K(+) currents in canine ventricular cardiomyocytes.Br J Pharmacol. 2011 Feb;162(4):890-6. doi: 10.1111/j.1476-5381.2010.01092.x. Br J Pharmacol. 2011. PMID: 20973780 Free PMC article.
-
Synergistic activation of guinea-pig cardiac cystic fibrosis transmembrane conductance regulator by the tyrosine kinase inhibitor genistein and cAMP.J Physiol. 1997 Nov 15;505 ( Pt 1)(Pt 1):23-40. doi: 10.1111/j.1469-7793.1997.023bc.x. J Physiol. 1997. PMID: 9409469 Free PMC article.
-
17beta-estradiol potentiates the cardiac cystic fibrosis transmembrane conductance regulator chloride current in guinea-pig ventricular myocytes.J Physiol Sci. 2006 Feb;56(1):29-37. doi: 10.2170/physiolsci.r2131. J Physiol Sci. 2006. PMID: 16779911
References
-
- Sorota S. Insights into the structure, distribution and function of the cardiac chloride channels. Cardiovasc. Res. 1999;42:361–376. - PubMed
-
- Mulvaney A.W., Spencer C.I., Culliford S., Borg J.J., Davies S.G., Kozlowski R.Z. Cardiac chloride channels: physiology, pharmacology and approaches for identifying novel modulators of activity. Drug Discov. Today. 2000;5:492–505. - PubMed
-
- Bahinski A., Gadsby D.C., Greengard P., Nairn A.C. Chloride conductance regulated by protein kinase A in isolated guinea-pig ventricular myocytes. J. Physiol. 1989;418:32P. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

