Rab11 regulates cell-cell communication during collective cell movements
- PMID: 23376974
- PMCID: PMC4006229
- DOI: 10.1038/ncb2681
Rab11 regulates cell-cell communication during collective cell movements
Abstract
Collective cell movements contribute to development and metastasis. The small GTPase Rac is a key regulator of actin dynamics and cell migration but the mechanisms that restrict Rac activation and localization in a group of collectively migrating cells are unknown. Here, we demonstrate that the small GTPases Rab5 and Rab11 regulate Rac activity and polarization during collective cell migration. We use photoactivatable forms of Rac to demonstrate that Rab11 acts on the entire group to ensure that Rac activity is properly restricted to the leading cell through regulation of cell-cell communication. In addition, we show that Rab11 binds to the actin cytoskeleton regulator Moesin and regulates its activation in vivo during migration. Accordingly, reducing the level of Moesin activity also affects cell-cell communication, whereas expressing active Moesin rescues loss of Rab11 function. Our model suggests that Rab11 controls the sensing of the relative levels of Rac activity in a group of cells, leading to the organization of individual cells in a coherent multicellular motile structure.
Figures


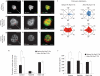
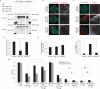

References
-
- Rorth P. Collective cell migration. Annu. Rev. Cell Dev. Biol. 2009;25:407–429. - PubMed
-
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol. Cancer Res. 2010;8:629–642. - PubMed
-
- Thiery JP. Metastasis: alone or together? Curr. Biol. 2009;19:R1121–R1123. - PubMed
-
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. - PubMed
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

