Forebrain HCN1 channels contribute to hypnotic actions of ketamine
- PMID: 23377220
- PMCID: PMC3605219
- DOI: 10.1097/ALN.0b013e318287b7c8
Forebrain HCN1 channels contribute to hypnotic actions of ketamine
Abstract
Background: Ketamine is a commonly used anesthetic, but the mechanistic basis for its clinically relevant actions remains to be determined. The authors previously showed that HCN1 channels are inhibited by ketamine and demonstrated that global HCN1 knockout mice are twofold less sensitive to hypnotic actions of ketamine. Although that work identified HCN1 channels as a viable molecular target for ketamine, it did not determine the relevant neural substrate.
Methods: To localize the brain region responsible for HCN1-mediated hypnotic actions of ketamine, the authors used a conditional knockout strategy to delete HCN1 channels selectively in excitatory cells of the mouse forebrain. A combination of molecular, immunohistochemical, and cellular electrophysiologic approaches was used to verify conditional HCN1 deletion; a loss-of-righting reflex assay served to ascertain effects of forebrain HCN1 channel ablation on hypnotic actions of ketamine.
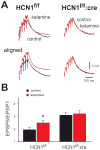
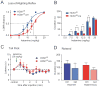
Results: In conditional knockout mice, HCN1 channels were selectively deleted in cortex and hippocampus, with expression retained in cerebellum. In cortical pyramidal neurons from forebrain-selective HCN1 knockout mice, effects of ketamine on HCN1-dependent membrane properties were absent; notably, ketamine was unable to evoke membrane hyperpolarization or enhance synaptic inputs. Finally, the EC50 for ketamine-induced loss-of-righting reflex was shifted to significantly higher concentrations (by approximately 31%).
Conclusions: These data indicate that forebrain principal cells represent a relevant neural substrate for HCN1-mediated hypnotic actions of ketamine. The authors suggest that ketamine inhibition of HCN1 shifts cortical neuron electroresponsive properties to contribute to ketamine-induced hypnosis.
Figures



References
-
- Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nature Rev Neurosci. 2008;9:370–86. - PubMed
-
- Grasshoff C, Rudolph U, Antkowiak B. Molecular and systemic mechanisms of general anaesthesia: the ‘multi-site and multiple mechanisms’ concept. Curr Opin Anaesthesiol. 2005;18:386–91. - PubMed
-
- Pape HC. Queer current and pacemaker: The hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

