Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC)
- PMID: 23377563
- PMCID: PMC3675273
- DOI: 10.1245/s10434-012-2840-2
Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Abstract
Background: Both in animal models and in human patients, pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been shown to improve local bioavailability of chemotherapy in peritoneal nodules, as compared with conventional peritoneal lavage. Pharmacokinetic studies show a low drug concentration in peripheral venous blood. However, hepatic and renal toxicities induced by delivering chemotherapeutic drugs into the abdomen as a pressurized aerosol have not yet been investigated.
Methods: Liver and renal function as well as toxicity parameters were monitored after eight PIPAC applications with doxorubicin (1.5 mg/m(2) body surface) and cisplatin (7.5 mg/m(2) body surface) in three end-stage patients suffering therapy-resistant peritoneal carcinomatosis. PIPAC was repeated at 4-week intervals (three times in two patients, twice in one patient). Peripheral venous blood was collected preoperatively and then daily until the 5th postoperative day, and sent to the hospital's clinical chemistry laboratory. Statistical analysis was performed by analysis of variance (ANOVA).
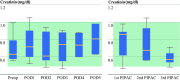
Results: Gamma-glutamyltransferase was significantly elevated (p < 0.05) in the early postoperative phase. Glutamic oxaloacetic transaminase [aspartate aminotransferase], glutamic pyruvic transaminase [alanine aminotransferase], and bilirubin levels were not influenced by the procedure. Quick-test remained normal. Serum creatinine levels were not altered.
Conclusions: Under the above conditions, PIPAC did not induce clinically relevant liver cytotoxicity. Liver metabolism and function were not altered. Renal function remained within the normal range. No cumulative toxicity was observed after repeated PIPAC. PIPAC appears to be associated with very limited hepatic and renal toxicity, which might be a significant advantage over other administration routes.
Figures

References
-
- Van Lierde S, Denys H, Peeters M. Systemic chemotherapy in patients with peritoneal carcinomatosis from non colorectal origin. Cancer Treat Res. 2007;134:441–448. - PubMed
-
- Zani S, Papalezova K, Stinnett S, et al. Modest advances in survival for patients with colorectal-associated peritoneal carcinomatosis in the era of modern chemotherapy. J Surg Oncol. 2012;18 Jul. Epub ahead of print. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

