Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients
- PMID: 23377664
- PMCID: PMC3568250
- DOI: 10.1097/CJI.0b013e3182811ae4
Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients
Abstract
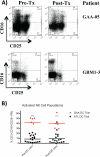
Dendritic cell (DC) vaccination is emerging as a promising therapeutic option for malignant glioma patients. However, the optimal antigen formulation for loading these cells has yet to be established. The objective of this study was to compare the safety, feasibility, and immune responses of malignant glioma patients on 2 different DC vaccination protocols. Twenty-eight patients were treated with autologous tumor lysate (ATL)-pulsed DC vaccination, whereas 6 patients were treated with glioma-associated antigen (GAA) peptide-pulsed DCs. Safety, toxicity, feasibility, and correlative immune monitoring assay results were compared between patients on each trial. Because of HLA subtype restrictions on the GAA-DC trial, 6/15 screened patients were eligible for treatment, whereas 28/32 patients passed eligibility screening for the ATL-DC trial. Elevated frequencies of activated natural killer cells were observed in the peripheral blood from GAA-DC patients compared with the ATL-DC patients. In addition, a significant correlation was observed between decreased regulatory T lymphocyte (Treg) ratios (postvaccination/prevaccination) and overall survival (P = 0.004) in patients on both trials. In fact, Treg ratios were independently prognostic for overall survival in these patients, whereas tumor pathology was not in multivariate analyses. In conclusion, these results suggest that ATL-DC vaccination is associated with wider patient eligibility compared with GAA-DC vaccination. Decreased postvaccination/prevaccination Treg ratios and decreased frequencies of activated natural killer cells were associated with prolonged survival in patients from both trials, suggesting that these lymphocyte subsets may be relevant immune monitoring endpoints for immunotherapy protocols in malignant glioma patients.
Trial registration: ClinicalTrials.gov NCT00068510 NCT00612001.
Figures

References
-
- Nieder C. Treatment of newly diagnosed glioblastoma multiforme. J Clin Oncol. 2002;20:3179–80. author reply 81-2. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Yang MY, Zetler PM, Prins RM, Khan-Farooqi H, Liau LM. Immunotherapy for patients with malignant glioma: from theoretical principles to clinical applications. Expert Rev Neurother. 2006;6:1481–94. - PubMed
-
- De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–104. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

