Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise
- PMID: 23377961
- PMCID: PMC3575690
- DOI: 10.1007/s00395-013-0332-6
Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise
Abstract
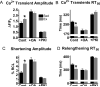
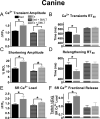
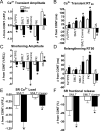
Exercise results in beneficial adaptations of the heart that can be directly observed at the ventricular myocyte level. However, the molecular mechanism(s) responsible for these adaptations are not well understood. Interestingly, signaling via neuronal nitric oxide synthase (NOS1) within myocytes results in similar effects as exercise. Thus, the objective was to define the role NOS1 plays in the exercise-induced beneficial contractile effects in myocytes. After an 8-week aerobic interval training program, exercise-trained (Ex) mice had higher VO(2max) and cardiac hypertrophy compared to sedentary (Sed) mice. Ventricular myocytes from Ex mice had increased NOS1 expression and nitric oxide production compared to myocytes from Sed mice. Remarkably, acute NOS1 inhibition normalized the enhanced contraction (shortening and Ca(2+) transients) in Ex myocytes to Sed levels. The NOS1 effect on contraction was mediated via greater Ca(2+) cycling that resulted from increased phospholamban phosphorylation. Intriguingly, a similar aerobic interval training program on NOS1 knockout mice failed to produce any beneficial cardiac adaptations (VO(2max), hypertrophy, and contraction). These data demonstrate that the beneficial cardiac adaptations observed after exercise training were mediated via enhanced NOS1 signaling. Therefore, it is likely that beneficial effects of exercise may be mimicked by the interventions that increase NOS1 signaling. This pathway may provide a potential novel therapeutic target in cardiac patients who are unable or unwilling to exercise.
Figures

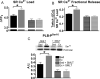

Similar articles
-
Diesterified nitrone rescues nitroso-redox levels and increases myocyte contraction via increased SR Ca(2+) handling.PLoS One. 2012;7(12):e52005. doi: 10.1371/journal.pone.0052005. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300588 Free PMC article.
-
Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1566-75. doi: 10.1152/ajpcell.00367.2007. Epub 2008 Apr 9. Am J Physiol Cell Physiol. 2008. PMID: 18400986 Free PMC article.
-
Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases.Circ Res. 2003 Jun 27;92(12):1322-9. doi: 10.1161/01.RES.0000078171.52542.9E. Epub 2003 May 22. Circ Res. 2003. PMID: 12764022
-
Cardiac myocyte neuronal nitric oxide synthase. New therapeutic target in heart failure?Arch Mal Coeur Vaiss. 2005 Dec;98(12):1244-8. Arch Mal Coeur Vaiss. 2005. PMID: 16435605 Review.
-
Abnormal Ca(2+) cycling in failing ventricular myocytes: role of NOS1-mediated nitroso-redox balance.Antioxid Redox Signal. 2014 Nov 10;21(14):2044-59. doi: 10.1089/ars.2014.5873. Epub 2014 Aug 7. Antioxid Redox Signal. 2014. PMID: 24801117 Free PMC article. Review.
Cited by
-
Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation.PLoS One. 2017 Jun 16;12(6):e0179648. doi: 10.1371/journal.pone.0179648. eCollection 2017. PLoS One. 2017. PMID: 28622359 Free PMC article.
-
Non-neuronal cardiac acetylcholine system playing indispensable roles in cardiac homeostasis confers resiliency to the heart.J Physiol Sci. 2021 Jan 18;71(1):2. doi: 10.1186/s12576-020-00787-6. J Physiol Sci. 2021. PMID: 33461483 Free PMC article.
-
Compromised redox homeostasis, altered nitroso-redox balance, and therapeutic possibilities in atrial fibrillation.Cardiovasc Res. 2016 Apr 1;109(4):510-8. doi: 10.1093/cvr/cvw012. Epub 2016 Jan 19. Cardiovasc Res. 2016. PMID: 26786158 Free PMC article. Review.
-
Expression levels of sarcolemmal membrane repair proteins following prolonged exercise training in mice.Indian J Biochem Biophys. 2013 Oct;50(5):428-35. Indian J Biochem Biophys. 2013. PMID: 24772964 Free PMC article.
-
Obligatory role of neuronal nitric oxide synthase in the heart's antioxidant adaptation with exercise.J Mol Cell Cardiol. 2015 Apr;81:54-61. doi: 10.1016/j.yjmcc.2015.01.003. Epub 2015 Jan 14. J Mol Cell Cardiol. 2015. PMID: 25595735 Free PMC article.
References
-
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416005a. - PubMed
-
- Bassett DR, Jr., Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. - PubMed
-
- Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3:187–204. doi: 10.1016/j.mito.2003.10.001. - PubMed
-
- Burkard N, Williams T, Czolbe M, Blomer N, Panther F, Link M, Fraccarollo D, Widder JD, Hu K, Han H, Hofmann U, Frantz S, Nordbeck P, Bulla J, Schuh K, Ritter O. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010;122:1588–1603. doi: 10.1161/CIRCULATIONAHA.109.933630. - PubMed
-
- Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci. 2008;13:312–324. doi: 10.2741/2681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

