At the leading edge of three-dimensional cell migration
- PMID: 23378019
- PMCID: PMC4067260
- DOI: 10.1242/jcs.093732
At the leading edge of three-dimensional cell migration
Abstract
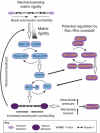
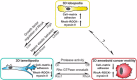
Cells migrating on flat two-dimensional (2D) surfaces use actin polymerization to extend the leading edge of the plasma membrane during lamellipodia-based migration. This mode of migration is not universal; it represents only one of several mechanisms of cell motility in three-dimensional (3D) environments. The distinct modes of 3D migration are strongly dependent on the physical properties of the extracellular matrix, and they can be distinguished by the structure of the leading edge and the degree of matrix adhesion. How are these distinct modes of cell motility in 3D environments related to each other and regulated? Recent studies show that the same type of cell migrating in 3D extracellular matrix can switch between different leading edge structures. This mode-switching behavior, or plasticity, by a single cell suggests that the apparent diversity of motility mechanisms is integrated by a common intracellular signaling pathway that governs the mode of cell migration. In this Commentary, we propose that the mode of 3D cell migration is governed by a signaling axis involving cell-matrix adhesions, RhoA signaling and actomyosin contractility, and that this might represent a universal mechanism that controls 3D cell migration.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

