ERdj3 regulates BiP occupancy in living cells
- PMID: 23378021
- PMCID: PMC3644143
- DOI: 10.1242/jcs.118182
ERdj3 regulates BiP occupancy in living cells
Abstract
Co-chaperones regulate chaperone activities and are likely to impact a protein-folding environment as much as the chaperone itself. As co-chaperones are expressed substoichiometrically, the ability of co-chaperones to encounter a chaperone is crucial for chaperone activity. ERdj3, an abundant soluble endoplasmic reticulum (ER) co-chaperone of the Hsp70 BiP, stimulates the ATPase activity of BiP to increase BiP's affinity for client (or substrate) proteins. We investigated ERdj3 availability, how ERdj3 levels impact BiP availability, and the significance of J proteins for regulating BiP binding of clients in living cells. FRAP analysis revealed that overexpressed ERdj3-sfGFP dramatically decreases BiP-GFP mobility in a client-dependent manner. By contrast, ERdj3-GFP mobility remains low regardless of client protein levels. Native gels and co-immunoprecipitations established that ERdj3 associates with a large complex including Sec61α. Translocon binding probably ensures rapid encounters between emerging nascent peptides and stimulates BiP activity in the crucial early stages of secretory protein folding. Importantly, mutant BiP exhibited significantly increased mobility when it could not interact with any ERdjs. Thus, ERdjs appear to play the dual roles of increasing BiP affinity for clients and regulating delivery of clients to BiP. Our data suggest that BiP engagement of clients is enhanced in ER subdomains enriched in ERdj proteins.
Keywords: BiP; ERdj; FRAP; Fluorescence recovery after photobleaching; Quality control; Superfolder GFP.
Figures
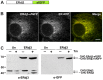

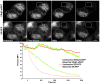
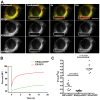



Similar articles
-
SDF2-like protein 1 (SDF2L1) regulates the endoplasmic reticulum localization and chaperone activity of ERdj3 protein.J Biol Chem. 2019 Dec 13;294(50):19335-19348. doi: 10.1074/jbc.RA119.009603. Epub 2019 Oct 17. J Biol Chem. 2019. PMID: 31624144 Free PMC article.
-
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends.J Biol Chem. 2019 Feb 8;294(6):2098-2108. doi: 10.1074/jbc.REV118.002804. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563838 Free PMC article. Review.
-
ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP's interactions with unfolded substrates.Mol Biol Cell. 2005 Jan;16(1):40-50. doi: 10.1091/mbc.e04-05-0434. Epub 2004 Nov 3. Mol Biol Cell. 2005. PMID: 15525676 Free PMC article.
-
Members of the Hsp70 Family Recognize Distinct Types of Sequences to Execute ER Quality Control.Mol Cell. 2016 Sep 1;63(5):739-52. doi: 10.1016/j.molcel.2016.07.012. Epub 2016 Aug 18. Mol Cell. 2016. PMID: 27546788 Free PMC article.
-
Mechanisms of Protein Quality Control in the Endoplasmic Reticulum by a Coordinated Hsp40-Hsp70-Hsp90 System.Annu Rev Biophys. 2023 May 9;52:509-524. doi: 10.1146/annurev-biophys-111622-091309. Annu Rev Biophys. 2023. PMID: 37159299 Review.
Cited by
-
Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface.J Biol Chem. 2015 Mar 27;290(13):8049-64. doi: 10.1074/jbc.M114.618736. Epub 2015 Feb 11. J Biol Chem. 2015. PMID: 25673690 Free PMC article.
-
Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis.EMBO J. 2015 Jan 2;34(1):4-19. doi: 10.15252/embj.201488896. Epub 2014 Oct 31. EMBO J. 2015. PMID: 25361606 Free PMC article.
-
Erdj3 Has an Essential Role for Z Variant Alpha-1-Antitrypsin Degradation.J Cell Biochem. 2017 Oct;118(10):3090-3101. doi: 10.1002/jcb.26069. Epub 2017 Jun 20. J Cell Biochem. 2017. PMID: 28419579 Free PMC article.
-
Reshaping endoplasmic reticulum quality control through the unfolded protein response.Mol Cell. 2022 Apr 21;82(8):1477-1491. doi: 10.1016/j.molcel.2022.03.025. Mol Cell. 2022. PMID: 35452616 Free PMC article. Review.
-
Probing endoplasmic reticulum dynamics using fluorescence imaging and photobleaching techniques.Curr Protoc Cell Biol. 2013 Sep 24;60:21.7.1-21.7.29. doi: 10.1002/0471143030.cb2107s60. Curr Protoc Cell Biol. 2013. PMID: 24510787 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

