CD317/tetherin is an organiser of membrane microdomains
- PMID: 23378022
- PMCID: PMC3647434
- DOI: 10.1242/jcs.112953
CD317/tetherin is an organiser of membrane microdomains
Abstract
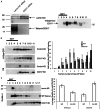
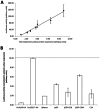
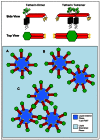
The integral membrane protein tetherin has been associated with an eclectic mix of cellular processes, including restricting the release of a range of enveloped viruses from infected cells. The unusual topology of tetherin (it possesses both a conventional transmembrane domain and a glycosylphosphatidylinositol anchor), its localisation to membrane microdomains (lipid rafts) and the fact that its cytosolic domain can be linked (indirectly) to the actin cytoskeleton, led us to speculate that tetherin might form a 'tethered picket fence' and thereby play a role in the organisation of lipid rafts. We now show that knocking down expression of tetherin leads to changes in the distribution of lipid raft-localised proteins and changes in the organisation of lipids in the plasma membrane. These changes can be reversed by re-expression of wild-type tetherin, but not by any of a range of tetherin-based constructs, indicating that no individual feature of the tetherin sequence is dispensable in the context of its lipid raft organising function.
Keywords: Laurdan; Lipid raft; Membrane; Tetherin.
Figures


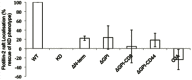
Similar articles
-
Expression of HIV-1 Vpu leads to loss of the viral restriction factor CD317/Tetherin from lipid rafts and its enhanced lysosomal degradation.PLoS One. 2013 Sep 24;8(9):e75680. doi: 10.1371/journal.pone.0075680. eCollection 2013. PLoS One. 2013. PMID: 24086611 Free PMC article.
-
Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif.J Cell Sci. 2007 Nov 1;120(Pt 21):3850-8. doi: 10.1242/jcs.003343. Epub 2007 Oct 16. J Cell Sci. 2007. PMID: 17940069
-
Anti-tetherin activities of HIV-1 Vpu and Ebola virus glycoprotein do not involve removal of tetherin from lipid rafts.J Virol. 2012 May;86(10):5467-80. doi: 10.1128/JVI.06280-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398279 Free PMC article.
-
Intracellular logistics of BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):321-6. doi: 10.2174/157016212800792522. Curr HIV Res. 2012. PMID: 22533471 Review.
-
Transmembrane interactions of HIV-1 Vpu and tetherin.Curr HIV Res. 2012 Jun;10(4):292-7. doi: 10.2174/157016212800792450. Curr HIV Res. 2012. PMID: 22524177 Review.
Cited by
-
BST-2/tetherin: viral tether, viral sensor or both?Future Virol. 2013 Nov;8(11):10.2217/fvl.13.96. doi: 10.2217/fvl.13.96. Future Virol. 2013. PMID: 24396393 Free PMC article.
-
BST2, a Novel Inhibitory Receptor, Is Involved in NK Cell Cytotoxicity through Its Cytoplasmic Tail Domain.Int J Mol Sci. 2022 Sep 27;23(19):11395. doi: 10.3390/ijms231911395. Int J Mol Sci. 2022. PMID: 36232695 Free PMC article.
-
From a gene-centric to whole-proteome view of differentiation of T helper cell subsets.Brief Funct Genomics. 2013 Nov;12(6):471-82. doi: 10.1093/bfgp/elt033. Epub 2013 Oct 7. Brief Funct Genomics. 2013. PMID: 24106101 Free PMC article.
-
The cytosolic N-terminus of CD317/tetherin is a membrane microdomain exclusion motif.Biol Open. 2013 Oct 15;2(11):1253-63. doi: 10.1242/bio.20135793. eCollection 2013. Biol Open. 2013. PMID: 24244863 Free PMC article.
-
Tetherin/BST-2 promotes dendritic cell activation and function during acute retrovirus infection.Sci Rep. 2016 Feb 5;6:20425. doi: 10.1038/srep20425. Sci Rep. 2016. PMID: 26846717 Free PMC article.
References
-
- Aranda J. F., Reglero-Real N., Kremer L., Marcos-Ramiro B., Ruiz-Sáenz A., Calvo M., Enrich C., Correas I., Millán J., Alonso M. A. (2011). MYADM regulates Rac1 targeting to ordered membranes required for cell spreading and migration. Mol. Biol. Cell 22, 1252–1262 10.1091/mbc.E10-11-0910 - DOI - PMC - PubMed
-
- Becker M., Sommer A., Krätzschmar J. R., Seidel H., Pohlenz H. D., Fichtner I. (2005). Distinct gene expression patterns in a tamoxifen-sensitive human mammary carcinoma xenograft and its tamoxifen-resistant subline MaCa 3366/TAM. Mol. Cancer Ther. 4, 151–168 - PubMed
-
- Blasius A. L., Giurisato E., Cella M., Schreiber R. D., Shaw A. S., Colonna M. (2006). Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177, 3260–3265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

