Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis
- PMID: 23378072
- PMCID: PMC3667714
- DOI: 10.1002/cm.21095
Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis
Abstract
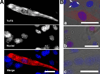
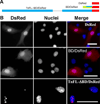
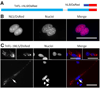
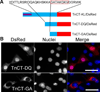
Troponin T (TnT) plays a major role in striated muscle contraction. We recently demonstrated that the fast skeletal muscle TnT3 isoform is localized in the muscle nucleus, and either its full-length or COOH-terminus leads to muscle cell apoptosis. Here, we further explored the mechanism by which it enters the nucleus and promotes cytotoxicity. Amino acid truncation and substitution showed that its COOH-terminus contains a dominant nuclear/nucleolar localization sequence (KLKRQK) and the basic lysine and arginine residues might play an important role in the nuclear retention and nucleolar enrichment of KLKRQK-DsRed fusion proteins. Deleting this domain or substituting lysine and arginine residues (KLAAQK) resulted in a dramatic loss of TnT3 nuclear and nucleolar localization. In contrast, the GATAKGKVGGRWK domain-DsRed construct localized exclusively in the cytoplasm, indicating that a nuclear exporting sequence is possibly localized in this region. Additionally, we identified a classical DNA-binding leucine zipper domain (LZD) which is conserved among TnT isoforms and species. Deletion of LZD or KLKRQK sequence significantly reduced cell apoptosis compared to full-length TnT3. We conclude that TnT3 contains both a nuclear localization signal and a DNA-binding domain, which may mediate nuclear/nucleolar signaling and muscle cell apoptosis.
Copyright © 2013 Wiley Periodicals, Inc.
Figures







Similar articles
-
Troponin T3 regulates nuclear localization of the calcium channel Cavβ1a subunit in skeletal muscle.Exp Cell Res. 2015 Aug 15;336(2):276-86. doi: 10.1016/j.yexcr.2015.05.005. Epub 2015 May 15. Exp Cell Res. 2015. PMID: 25981458 Free PMC article.
-
Troponin T nuclear localization and its role in aging skeletal muscle.Age (Dordr). 2013 Apr;35(2):353-70. doi: 10.1007/s11357-011-9368-4. Epub 2011 Dec 22. Age (Dordr). 2013. PMID: 22189912 Free PMC article.
-
Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs.Exp Gerontol. 2018 Jul 15;108:35-40. doi: 10.1016/j.exger.2018.03.012. Epub 2018 Mar 27. Exp Gerontol. 2018. PMID: 29596868 Free PMC article.
-
Calpain inhibition rescues troponin T3 fragmentation, increases Cav1.1, and enhances skeletal muscle force in aging sedentary mice.Aging Cell. 2016 Jun;15(3):488-98. doi: 10.1111/acel.12453. Epub 2016 Feb 19. Aging Cell. 2016. PMID: 26892246 Free PMC article.
-
TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships.Gene. 2016 May 10;582(1):1-13. doi: 10.1016/j.gene.2016.01.006. Epub 2016 Jan 13. Gene. 2016. PMID: 26774798 Free PMC article. Review.
Cited by
-
Nuclear tropomyosin and troponin in striated muscle: new roles in a new locale?J Muscle Res Cell Motil. 2013 Aug;34(3-4):275-84. doi: 10.1007/s10974-013-9356-7. Epub 2013 Aug 2. J Muscle Res Cell Motil. 2013. PMID: 23907338 Review.
-
Non-Canonical Localization of Cardiac Troponins: Expanding Functions or Causing Pathologies?Int J Mol Sci. 2024 Mar 8;25(6):3117. doi: 10.3390/ijms25063117. Int J Mol Sci. 2024. PMID: 38542091 Free PMC article. Review.
-
Will you still need me (Ca2+ , TnT, and DHPR), will you still cleave me (calpain), when I'm 64?Aging Cell. 2017 Apr;16(2):202-204. doi: 10.1111/acel.12560. Epub 2016 Dec 23. Aging Cell. 2017. PMID: 28008709 Free PMC article. No abstract available.
-
Cardiac troponin T and autoimmunity in skeletal muscle aging.Geroscience. 2022 Aug;44(4):2025-2045. doi: 10.1007/s11357-022-00513-7. Epub 2022 Jan 15. Geroscience. 2022. PMID: 35034279 Free PMC article.
-
Troponin T3 regulates nuclear localization of the calcium channel Cavβ1a subunit in skeletal muscle.Exp Cell Res. 2015 Aug 15;336(2):276-86. doi: 10.1016/j.yexcr.2015.05.005. Epub 2015 May 15. Exp Cell Res. 2015. PMID: 25981458 Free PMC article.
References
-
- Asumda FZ, Chase PB. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation. 2012;83(3):106–115. - PubMed
-
- Banfic H, Visnjic D, Mise N, Balakrishnan S, Deplano S, Korchev YE, Domin J. Epidermal growth factor stimulates translocation of the class II phosphoinositide 3-kinase PI3K-C2beta to the nucleus. Biochem J. 2009;422(1):53–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

