Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women
- PMID: 23378284
- PMCID: PMC3634309
- DOI: 10.1093/cid/cit057
Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women
Abstract
Background: Pregnant women infected with human immunodeficiency virus (HIV) may have particular vulnerability to 2009 pandemic H1N1 influenza (pH1N1) infection. The safety and immunogenicity of pH1N1 vaccination in HIV-infected pregnant women are unknown.
Methods: HIV-infected women 18-39 years of age and 14-34 weeks' gestation on antiretroviral therapy received two 30-μg doses of unadjuvanted, inactivated pH1N1 vaccine 21 days apart. Hemagglutination inhibition titers were measured at entry, 21 days after dose 1, and 10 and 21 days after dose 2, and, in mothers and infants, at delivery and 3 and 6 months postdelivery.
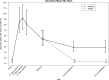
Results: No severe vaccine-related adverse events were observed among 127 subjects. At entry, 21% had seroprotective (≥1:40) titers. Seroprotection and seroresponse (≥4-fold rise) occurred in 73% and 66% after dose 1 and 80% and 72% after dose 2, respectively. Of women lacking seroprotection at entry, 66% attained seroprotection after dose 1 and 75% after dose 2. Seroprotective titers were present in 67% of mothers and 65% of infants at delivery (median 66 days after dose 2), 60% of mothers and 26% of infants at 3 months postdelivery, and 59% of mothers and 12% of infants at 6 months postdelivery.
Conclusions: Two 30-μg doses were moderately immunogenic in HIV-infected pregnant women. No concerning vaccine-related safety signals were observed. Seroprotection persisted in most women postpartum. Efficient transplacental antibody transfer occurred, but seroprotection in infants waned rapidly. Vaccination to protect HIV-infected pregnant women and their newborns from new influenza strains is feasible, but more immunogenic platforms should be evaluated. Clinical Trials Registration. NCT00992017.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- N01-DK-9-001/DK/NIDDK NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- RR00069/RR/NCRR NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

