Evidence for postinitiation regulation of mRNA biogenesis in tuberculosis
- PMID: 23378427
- PMCID: PMC3711638
- DOI: 10.4049/jimmunol.1202185
Evidence for postinitiation regulation of mRNA biogenesis in tuberculosis
Abstract
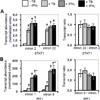
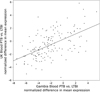
Mycobacterium tuberculosis infection alters macrophage gene expression and macrophage response to IFN-γ, a critical host defense cytokine. However, regulation of these changes is poorly understood. We report discordance of changes in nascent transcript and total nuclear RNA abundance for the transcription factors STAT1 and IRF1, together with lack of effect on their RNA half-lives, in human THP-1 cells infected with M. tuberculosis and stimulated with IFN-γ. The results indicate that negative postinitiation regulation of mRNA biogenesis limits the expression of these factors, which mediate host defense against M. tuberculosis through the cellular response to IFN-γ. Consistent with the results for STAT1 and IRF1, transcriptome analysis reveals downregulation of postinitiation mRNA biogenesis processes and pathways by infection, with and without IFN-γ stimulation. Clinical relevance for regulation of postinitiation mRNA biogenesis is demonstrated by studies of donor samples showing that postinitiation mRNA biogenesis pathways are repressed in latent tuberculosis infection compared with cured disease and in active tuberculosis compared with ongoing treatment or with latent tuberculosis. For active disease and latent infection donors from two populations (London, U.K., and The Gambia), each analyzed using a different platform, pathway-related gene expression differences were highly correlated, demonstrating substantial specificity in the effect. Collectively, the molecular and bioinformatic analyses point toward downregulation of postinitiation mRNA biogenesis pathways as a means by which M. tuberculosis infection limits expression of immunologically essential transcription factors. Thus, negative regulation of postinitiation mRNA biogenesis can constrain the macrophage response to infection and overall host defense against tuberculosis.
Figures





References
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003;163:1009–1021. - PubMed
-
- Enarson DA, Chiang C-Y, Murray, F J. Global Epoidemiology of Tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. second ed. New York: Lippincott Williams & Wilkins; 2004. pp. 13–29.
-
- Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin. Infect. Dis. 2007;45:1290–1295. - PubMed
-
- Sotgiu G, Ferrara G, Matteelli A, Richardson MD, Centis R, Ruesch-Gerdes S, Toungoussova O, Zellweger JP, Spanevello A, Cirillo D, Lange C, Migliori GB. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur. Respir. J. 2009;33:871–881. - PubMed
-
- Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

