Ultrastructural analysis of the rugose cell envelope of a member of the Pasteurellaceae family
- PMID: 23378507
- PMCID: PMC3624570
- DOI: 10.1128/JB.02149-12
Ultrastructural analysis of the rugose cell envelope of a member of the Pasteurellaceae family
Abstract
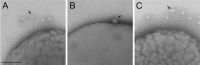
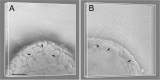
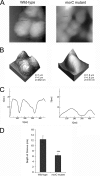
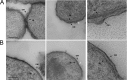
Bacterial membranes serve as selective environmental barriers and contain determinants required for bacterial colonization and survival. Cell envelopes of Gram-negative bacteria consist of an outer and an inner membrane separated by a periplasmic space. Most Gram-negative bacteria display a smooth outer surface (e.g., Enterobacteriaceae), whereas members of the Pasteurellaceae and Moraxellaceae families show convoluted surfaces. Aggregatibacter actinomycetemcomitans, an oral pathogen representative of the Pasteurellaceae family, displays a convoluted membrane morphology. This phenotype is associated with the presence of morphogenesis protein C (MorC). Inactivation of the morC gene results in a smooth membrane appearance when visualized by two-dimensional (2D) electron microscopy. In this study, 3D electron microscopy and atomic force microscopy of whole-mount bacterial preparations as well as 3D electron microscopy of ultrathin sections of high-pressure frozen and freeze-substituted specimens were used to characterize the membranes of both wild-type and morC mutant strains of A. actinomycetemcomitans. Our results show that the mutant strain contains fewer convolutions than the wild-type bacterium, which exhibits a higher curvature of the outer membrane and a periplasmic space with 2-fold larger volume/area ratio than the mutant bacterium. The inner membrane of both strains has a smooth appearance and shows connections with the outer membrane, as revealed by visualization and segmentation of 3D tomograms. The present studies and the availability of genetically modified organisms with altered outer membrane morphology make A. actinomycetemcomitans a model organism for examining membrane remodeling and its implications in antibiotic resistance and virulence in the Pasteurellaceae and Moraxellaceae bacterial families.
Figures


Similar articles
-
The conserved carboxyl domain of MorC, an inner membrane protein of Aggregatibacter actinomycetemcomitans, is essential for membrane function.Mol Oral Microbiol. 2016 Feb;31(1):43-58. doi: 10.1111/omi.12120. Epub 2015 Sep 15. Mol Oral Microbiol. 2016. PMID: 26205976 Free PMC article.
-
O-polysaccharide glycosylation is required for stability and function of the collagen adhesin EmaA of Aggregatibacter actinomycetemcomitans.Infect Immun. 2012 Aug;80(8):2868-77. doi: 10.1128/IAI.00372-12. Epub 2012 Jun 11. Infect Immun. 2012. PMID: 22689812 Free PMC article.
-
QseBC, a two-component bacterial adrenergic receptor and global regulator of virulence in Enterobacteriaceae and Pasteurellaceae.Mol Oral Microbiol. 2016 Oct;31(5):379-97. doi: 10.1111/omi.12138. Epub 2015 Nov 20. Mol Oral Microbiol. 2016. PMID: 26426681 Free PMC article. Review.
-
Membrane morphology and leukotoxin secretion are associated with a novel membrane protein of Aggregatibacter actinomycetemcomitans.J Bacteriol. 2008 Sep;190(17):5972-80. doi: 10.1128/JB.00548-08. Epub 2008 Jul 11. J Bacteriol. 2008. PMID: 18621903 Free PMC article.
-
Recent approaches to the chemotaxonomy of the Actinobacillus-Haemophilus-Pasteurella group (family Pasteurellaceae).Oral Microbiol Immunol. 1993 Dec;8(6):327-36. doi: 10.1111/j.1399-302x.1993.tb00607.x. Oral Microbiol Immunol. 1993. PMID: 7512257 Review.
Cited by
-
The antibacterial effects of vitamin D3 against mutans streptococci: an in vitro study.Eur Oral Res. 2021 Jan 4;55(1):8-15. doi: 10.26650/eor.20210119. Eur Oral Res. 2021. PMID: 33937756 Free PMC article.
-
The conserved carboxyl domain of MorC, an inner membrane protein of Aggregatibacter actinomycetemcomitans, is essential for membrane function.Mol Oral Microbiol. 2016 Feb;31(1):43-58. doi: 10.1111/omi.12120. Epub 2015 Sep 15. Mol Oral Microbiol. 2016. PMID: 26205976 Free PMC article.
-
Antibacterial effectiveness of different zinc salts on Streptococcus mutans and Streptococcus sobrinus: An in-vitro study.Saudi Dent J. 2023 Nov;35(7):883-890. doi: 10.1016/j.sdentj.2023.07.003. Epub 2023 Jul 6. Saudi Dent J. 2023. PMID: 38025600 Free PMC article.
-
YhdP, TamB, and YdbH Are Redundant but Essential for Growth and Lipid Homeostasis of the Gram-Negative Outer Membrane.mBio. 2021 Dec 21;12(6):e0271421. doi: 10.1128/mBio.02714-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781743 Free PMC article.
-
Inner-membrane protein MorC is involved in fimbriae production and biofilm formation in Aggregatibacter actinomycetemcomitans.Microbiology (Reading). 2016 Mar;162(3):513-525. doi: 10.1099/mic.0.000246. Epub 2016 Jan 20. Microbiology (Reading). 2016. PMID: 26796329 Free PMC article.
References
-
- Graham LL, Beveridge TJ, Nanninga N. 1991. Periplasmic space and the concept of the periplasm. Trends Biochem. Sci. 16:328–329 - PubMed
-
- Glauert AM, Thornley MJ. 1969. The topography of the bacterial cell wall. Annu. Rev. Microbiol. 23:159–198 - PubMed
-
- Miura T, Mizushima S. 1968. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K-12. Biochim. Biophys. Acta 150:159–161 - PubMed
-
- Osborn MJ, Gander JE, Parisi E, Carson J. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium: isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 247:3962–3972 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

