Mating pair formation homologue TraG is a variable membrane protein essential for contact-independent type IV secretion of chromosomal DNA by Neisseria gonorrhoeae
- PMID: 23378511
- PMCID: PMC3624559
- DOI: 10.1128/JB.02098-12
Mating pair formation homologue TraG is a variable membrane protein essential for contact-independent type IV secretion of chromosomal DNA by Neisseria gonorrhoeae
Abstract
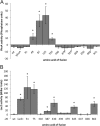
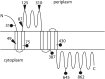
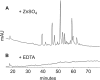
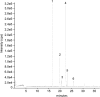
Neisseria gonorrhoeae uses a type IV secretion system (T4SS) to secrete chromosomal DNA into the surrounding milieu. The DNA is effective in transforming gonococci in the population, and this mechanism of DNA donation may contribute to the high degree of genetic diversity in this species. Similar to other F-like T4SSs, the gonococcal T4SS requires a putative membrane protein, TraG, for DNA transfer. In F-plasmid and related systems, the homologous protein acts in pilus production, mating pair stabilization, and entry exclusion. We characterized the localization, membrane topology, and variation of TraG in N. gonorrhoeae. TraG was found to be an inner-membrane protein with one large periplasmic region and one large cytoplasmic region. Each gonococcal strain carried one of three different alleles of traG. Strains that carried the smallest allele of traG were found to lack the peptidoglycanase gene atlA but carried a peptidoglycan endopeptidase gene in place of atlA. The purified endopeptidase degraded gonococcal peptidoglycan in vitro, cutting the peptide cross-links. Although the other two traG alleles functioned for DNA secretion in strain MS11, the smallest traG did not support DNA secretion. Despite the requirement for a mating pair stabilization homologue, static coculture transformation experiments demonstrated that DNA transfer was nuclease sensitive and required active uptake by the recipient, thus demonstrating that transfer occurred by transformation and not conjugation. Together, these results demonstrate the TraG acts in a process of DNA export not specific to conjugation and that different forms of TraG affect what substrates can be transported.
Figures





References
-
- Dillard JP, Seifert HS. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263–277 - PubMed
-
- Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 55:1704–1721 - PubMed
-
- Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59:376–385 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

