RNF13, a RING finger protein, mediates endoplasmic reticulum stress-induced apoptosis through the inositol-requiring enzyme (IRE1α)/c-Jun NH2-terminal kinase pathway
- PMID: 23378536
- PMCID: PMC3605690
- DOI: 10.1074/jbc.M112.368829
RNF13, a RING finger protein, mediates endoplasmic reticulum stress-induced apoptosis through the inositol-requiring enzyme (IRE1α)/c-Jun NH2-terminal kinase pathway
Abstract
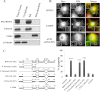
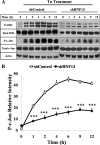
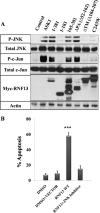
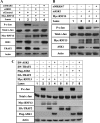
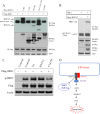
Disturbance of homeostasis at endoplasmic reticulum (ER) causes stress to cells that in turn triggers an adaptive signaling pathway termed unfolded protein response for the purpose of restoring normal cellular physiology or initiating signaling events leading to apoptosis. Identification of those genes that are involved in the unfolded protein response-mediated apoptotic signaling pathway would be valuable toward elucidating the molecular mechanism underlying the relationship between ER stress and apoptosis. We initiated a genetic screen by using the retroviral insertion mutation system to search for genes whose inactivation confers resistance to apoptosis induction by staurosporine. Using this approach, RING finger protein 13 (RNF13) was identified. Interestingly, RNF13 is highly enriched in ER. RNF13 knockdown cells are resistant to apoptosis and JNK activation triggered by ER stress. Conversely, overexpression of RNF13 induces JNK activation and caspase-dependent apoptosis. The RING and transmembrane domains of RNF13 are both required for its effects on JNK activation and apoptosis. Moreover, systematic analysis of the involvement of individual signaling components in the ER stress pathway using knockdown approach reveals that RNF13 acts upstream of the IRE1α-TRAF2 signaling axis for JNK activation and apoptosis. Finally, RNF13 co-immunoprecipitates with IRE1α, and the intact RING domain is also required for mediating its interaction. Together, our data support a model that RNF13 is a critical mediator for facilitating ER stress-induced apoptosis through the activation of the IRE1α-TRAF2-JNK signaling pathway.
Figures


References
-
- Kim I., Xu W., Reed J. C. (2008) Cell death and endoplasmic reticulum stress. Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013–1030 - PubMed
-
- Walter P., Ron D. (2011) The unfolded protein response. From stress pathway to homeostatic regulation. Science 334, 1081–1086 - PubMed
-
- Cnop M., Foufelle F., Velloso L. A. (2012) Endoplasmic reticulum stress, obesity, and diabetes. Trends Mol. Med. 18, 59–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

