Role of the gut microbiota in the development and function of lymphoid cells
- PMID: 23378581
- PMCID: PMC3564600
- DOI: 10.4049/jimmunol.1203100
Role of the gut microbiota in the development and function of lymphoid cells
Abstract
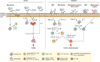
Mammals are colonized by large numbers of microorganisms, including trillions of bacteria, most of which live in the intestinal tract. These indigenous microorganisms that inhabit the body of humans and animals are referred collectively to as the microbiota. Accumulating evidence indicates that the microbiota regulates the development and/or function of different types of immune cells in the intestine. For example, the microbiota drives homeostatic, pathogenic, and regulatory T cell immune responses that contribute to tissue homeostasis, but also can promote disease. The gut microbes also facilitate IgA responses, which in turn regulate the composition and function of the gut microbiota. Thus, the reciprocal regulation of the gut microbiota and the host immune system may influence the balance between homeostasis and disease in the intestine.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
References
-
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. - PubMed
-
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. - PubMed
-
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

