A generalized G-SFED continuum solvation free energy calculation model
- PMID: 23378634
- PMCID: PMC3581891
- DOI: 10.1073/pnas.1221940110
A generalized G-SFED continuum solvation free energy calculation model
Abstract
An empirical continuum solvation model, solvation free energy density (SFED), has been developed to calculate solvation free energies of a molecule in the most frequently used solvents. A generalized version of the SFED model, generalized-SFED (G-SFED), is proposed here to calculate molecular solvation free energies in virtually any solvent. G-SFED provides an accurate and fast generalized framework without a complicated description of a solution. In the model, the solvation free energy of a solute is represented as a linear combination of empirical functions of the solute properties representing the effects of solute on various solute-solvent interactions, and the complementary solvent effects on these interactions were reflected in the linear expansion coefficients with a few solvent properties. G-SFED works well for a wide range of sizes and polarities of solute molecules in various solvents as shown by a set of 5,753 solvation free energies of diverse combinations of 103 solvents and 890 solutes. Octanol-water partition coefficients of small organic compounds and peptides were calculated with G-SFED with accuracy within 0.4 log unit for each group. The G-SFED computation time depends linearly on the number of nonhydrogen atoms (n) in a molecule, O(n).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
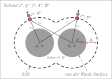
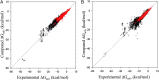
 . Red and black dots represent the solvation free energies in the training and external validation sets, respectively. The MAEs and R2s were 0.73 kcal/cal and 0.95, respectively, for G-SFED, and 0.90 kcal/mol and 0.89, respectively, for SM.42R.
. Red and black dots represent the solvation free energies in the training and external validation sets, respectively. The MAEs and R2s were 0.73 kcal/cal and 0.95, respectively, for G-SFED, and 0.90 kcal/mol and 0.89, respectively, for SM.42R.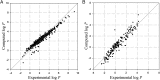
Similar articles
-
Incorporation of Hydrogen Bond Angle Dependency into the Generalized Solvation Free Energy Density Model.J Chem Inf Model. 2018 Apr 23;58(4):761-772. doi: 10.1021/acs.jcim.7b00410. Epub 2018 Mar 30. J Chem Inf Model. 2018. PMID: 29561152
-
A partition coefficient calculation method with the SFED model.J Chem Inf Model. 2005 Mar-Apr;45(2):254-63. doi: 10.1021/ci0498564. J Chem Inf Model. 2005. PMID: 15807486
-
Calculation of the solvation free energy of neutral and ionic molecules in diverse solvents.J Chem Inf Model. 2011 Jan 24;51(1):105-14. doi: 10.1021/ci100299m. Epub 2010 Dec 6. J Chem Inf Model. 2011. PMID: 21133372
-
Relationship between Solvation Thermodynamics from IST and DFT Perspectives.J Phys Chem B. 2017 Apr 20;121(15):3825-3841. doi: 10.1021/acs.jpcb.6b12889. Epub 2017 Feb 28. J Phys Chem B. 2017. PMID: 28186751 Free PMC article. Review.
-
Biomolecular Simulations with the Three-Dimensional Reference Interaction Site Model with the Kovalenko-Hirata Closure Molecular Solvation Theory.Int J Mol Sci. 2021 May 11;22(10):5061. doi: 10.3390/ijms22105061. Int J Mol Sci. 2021. PMID: 34064655 Free PMC article. Review.
Cited by
-
Structure-Based Understanding of Binding Affinity and Mode of Estrogen Receptor α Agonists and Antagonists.PLoS One. 2017 Jan 6;12(1):e0169607. doi: 10.1371/journal.pone.0169607. eCollection 2017. PLoS One. 2017. PMID: 28061508 Free PMC article.
-
Free-energy calculations for semi-flexible macromolecules: applications to DNA knotting and looping.J Chem Phys. 2014 Nov 7;141(17):174902. doi: 10.1063/1.4900657. J Chem Phys. 2014. PMID: 25381542 Free PMC article.
-
3D-QSAR study of steroidal and azaheterocyclic human aromatase inhibitors using quantitative profile of protein-ligand interactions.J Cheminform. 2018 Jan 18;10(1):2. doi: 10.1186/s13321-017-0253-8. J Cheminform. 2018. PMID: 29349513 Free PMC article.
-
PMFF: Development of a Physics-Based Molecular Force Field for Protein Simulation and Ligand Docking.J Phys Chem B. 2020 Feb 13;124(6):974-989. doi: 10.1021/acs.jpcb.9b10339. Epub 2020 Jan 29. J Phys Chem B. 2020. PMID: 31939671 Free PMC article.
References
-
- Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2003;20(2-3):153–214. - PubMed
-
- Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: Form follows function. Nat Rev Drug Discov. 2012;11(1):37–51. - PubMed
-
- Ángyán JG. Common theoretical framework for quantum chemical solvent effect theories. J Meth Phys. 1992;10:93–137.
-
- Tomasi J, Persico M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem Rev. 1994;94(7):2027–2094.
-
- Smith PE, Pettitt BM. Modeling solvent in biomolecular systems. J Phys Chem. 1994;98(39):9700–9711.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

