The effect of intravitreal bevacizumab and ranibizumab on cutaneous tensile strength during wound healing
- PMID: 23378736
- PMCID: PMC3559083
- DOI: 10.2147/OPTH.S40537
The effect of intravitreal bevacizumab and ranibizumab on cutaneous tensile strength during wound healing
Abstract
Purpose: To investigate the effect of intravitreal bevacizumab and ranibizumab on wound tension and by histopathology during cutaneous wound healing in a rabbit model and to compare this effect to placebo intravitreal saline controls 1 and 2 weeks following intravitreal injection.
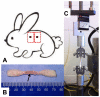
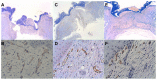
Methods: A total of 120 New Zealand white rabbits were randomly assigned to one of three treatment groups each consisting of 40 rabbits. Each group received intravitreal injections of bevacizumab, ranibizumab, or normal saline. Immediately afterwards, each rabbit underwent four 6 mm full-thickness dermatologic punch biopsies. Twenty rabbits from each agent group underwent wound harvesting on day 7 or day 14. The skin samples were stained for CD34 for vascular endothelial cells on day 7, and maximal wound tensile load was measured on days 7 and 14. Quantitative assessment of mean neovascularization (MNV) scores was obtained from 10 contiguous biopsy margin 400× fields of CD34-stained sections by two independent observers.
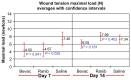
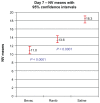
Results: Wound tension reading means (N) with standard error and adjusted P-values on day 7 were: saline placebos, 7.46 ± 0.87; bevacizumab, 4.50 ± 0.88 (P = 0.041); and ranibizumab, 4.67 ± 0.84 (P = 0.025). On day 14 these were: saline placebos, 7.34 ± 0.55; bevacizumab, 6.05 ± 0.54 (P = 0.18); and ranibizumab 7.99 ± 0.54 (P = 0.40). MNV scores in CD34 stained sections were: saline controls, 18.31 ± 0.43; bevacizumab, 11.02 ± 0.45 (P < 0.0001); and ranibizumab, 13.55 ± 0.43 (P < 0.0001). The interobserver correlation coefficient was 0.928.
Conclusion: At day 7, both anti-vascular endothelial growth factor (anti-VEGF) agents had significantly suppressed MNV scores and exerted a significant reduction of cutaneous wound tensile strength compared with saline controls. At day 14, neither agent produced a significant effect on tensile wound strength. Since angiogenesis is an integral component of the proliferative phase of wound healing, we encourage clinicians to be aware of their patients' recent surgical history during intravitreal anti-VEGF therapy and to consider refraining from their use during the perioperative period.
Keywords: bevacizumab; ranibizumab; tensile strength; wound healing.
Figures
References
-
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistic 2008 Update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. - PubMed
-
- Pouessel D, Culine S. High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur Urol. 2008;53(2):376–381. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

