Novel genetically-humanized mouse model established to evaluate efficacy of therapeutic agents to human interleukin-6 receptor
- PMID: 23378927
- PMCID: PMC3561642
- DOI: 10.1038/srep01196
Novel genetically-humanized mouse model established to evaluate efficacy of therapeutic agents to human interleukin-6 receptor
Abstract
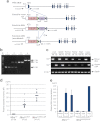
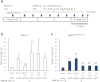
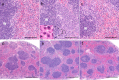
For clinical trials of therapeutic monoclonal antibodies (mAbs) to be successful, their efficacy needs to be adequately evaluated in preclinical experiments. However, in many cases it is difficult to evaluate the candidate mAbs using animal disease models because of lower cross-reactivity to the orthologous target molecules. In this study we have established a novel humanized Castleman's disease mouse model, in which the endogenous interleukin-6 receptor gene is successfully replaced by human IL6R, and human IL6 is overexpressed. We have also demonstrated the therapeutic effects of an antibody that neutralizes human IL6R, tocilizumab, on the symptoms in this mouse model. Plasma levels of human soluble IL6R and human IL6 were elevated after 4-week treatment of tocilizumab in this mouse model similarly to the result previously reported in patients treated with tocilizumab. Our mouse model provides us with a novel means of evaluating the in vivo efficacy of human IL6R-specific therapeutic agents.
Figures


Similar articles
-
[Pharmacological and clinical profile of anti-human IL-6 receptor antibody (tocilizumab, ACTEMRA), a novel therapeutic drug for Castleman's disease].Nihon Yakurigaku Zasshi. 2005 Dec;126(6):419-25. doi: 10.1254/fpj.126.419. Nihon Yakurigaku Zasshi. 2005. PMID: 16462093 Review. Japanese. No abstract available.
-
Short-term efficacy of the IL6 receptor antibody tocilizumab in patients with HIV-associated multicentric Castleman disease: report of two cases.J Hematol Oncol. 2014 Jan 17;7:10. doi: 10.1186/1756-8722-7-10. J Hematol Oncol. 2014. PMID: 24438824 Free PMC article.
-
Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman's disease.Intern Med. 2007;46(11):771-4. doi: 10.2169/internalmedicine.46.6262. Epub 2007 Jun 1. Intern Med. 2007. PMID: 17541233
-
Interleukin-6 in idiopathic multicentric Castleman's disease after long-term tocilizumab.Ann Hematol. 2017 Dec;96(12):2117-2119. doi: 10.1007/s00277-017-3111-x. Epub 2017 Sep 1. Ann Hematol. 2017. PMID: 28861599 No abstract available.
-
Anti-interleukin 6 receptor antibody treatment in rheumatic disease.Ann Rheum Dis. 2000 Nov;59 Suppl 1(Suppl 1):i21-7. doi: 10.1136/ard.59.suppl_1.i21. Ann Rheum Dis. 2000. PMID: 11053081 Free PMC article. Review.
Cited by
-
Generation of a transgenic mouse line for conditional expression of human IL-6.Exp Anim. 2016 Nov 1;65(4):455-463. doi: 10.1538/expanim.16-0043. Epub 2016 Jun 28. Exp Anim. 2016. PMID: 27349442 Free PMC article.
-
No evidence for paradoxical effects of tocilizumab in rodents.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):11061-11064. doi: 10.1007/s00210-025-04021-1. Epub 2025 Mar 12. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40072557 Free PMC article.
-
Mouse models of breast cancer.Methods Mol Biol. 2015;1267:47-71. doi: 10.1007/978-1-4939-2297-0_3. Methods Mol Biol. 2015. PMID: 25636464 Free PMC article.
-
Tocilizumab does not block interleukin-6 (IL-6) signaling in murine cells.PLoS One. 2020 May 4;15(5):e0232612. doi: 10.1371/journal.pone.0232612. eCollection 2020. PLoS One. 2020. PMID: 32365119 Free PMC article.
-
Phosphorylation of Hsp20 Promotes Fibrotic Remodeling and Heart Failure.JACC Basic Transl Sci. 2019 Apr 29;4(2):188-199. doi: 10.1016/j.jacbts.2018.11.007. eCollection 2019 Apr. JACC Basic Transl Sci. 2019. PMID: 31061921 Free PMC article.
References
-
- Loisel S. et al. Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment. Crit Rev Oncol Hematol. 62, 34–42 (2007). - PubMed
-
- Chapman K., Pullen N., Graham M. & Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nature Rev. Drud Discov. 6, 120–126 (2007). - PubMed
-
- Hansel T. T., Kropshofer H., Singer T., Mitchell J. A. & George A. J. The safety and side effects of monoclonal antibodies. Nature Rev. Drug Discov. 9, 325–338 (2010). - PubMed
-
- Palestro G., Turrini F., Pagano M. & Chiusa L. Castleman's disease. Adv. Clin. Path. 3, 11–22 (1999). - PubMed
-
- Cronin D. M. P. & Warnke R. A. Castleman disease: an update on classification and the spectrum of associated lesions. Adv. Anat. Pathol. 16, 236–246 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

