Differential effect of hyperglycaemia on the immune response in an experimental model of diabetes in BALB/cByJ and C57Bl/6J mice: participation of oxidative stress
- PMID: 23379439
- PMCID: PMC3569540
- DOI: 10.1111/cei.12020
Differential effect of hyperglycaemia on the immune response in an experimental model of diabetes in BALB/cByJ and C57Bl/6J mice: participation of oxidative stress
Abstract
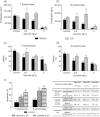
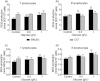
Diabetes is associated with an increased risk of death from infectious disease. Hyperglycaemia has been identified as the main factor contributing to the development of diseases associated with diabetes mellitus. However, experimental evidence indicates individual susceptibility to develop complications of diabetes. In this context, the aim of this work was to study the immune response in a streptozotocin-induced type 1 diabetes in two mouse strains: BALB/cByJ and C57Bl/6J. The participation of hyperglycaemia and oxidative stress was also analysed. Diabetic BALB/cByJ mice showed a decrease in both the in-vivo and in-vitro immune responses, whereas diabetic C57Bl/6J mice had higher blood glucose but exhibited no impairment of the immune response. The influence of hyperglycaemia over the immune response was evaluated by preincubation of lymphocytes from normal mice in a high glucose-containing medium. T and B cells from BALB/cByJ mice showed a decrease in cell viability and mitogen-stimulated proliferation and an increase in apoptosis induction. An increase in oxidative stress was implicated in this deleterious effect. These parameters were not affected in the T and B lymphocytes from C57Bl/6J mice. In conclusion, BALB/cByJ mice were sensitive to the deleterious effect of hyperglycaemia, while C57BL/6J were resistant. Although an extrapolation of these results to clinical conditions must be handled with caution, these results highlight the need to contemplate the genetic background to establish models to study the deleterious effect of diabetes in order to understand phenotypical variations that are of clinical importance in the treatment of patients.
© 2012 British Society for Immunology.
Figures





Similar articles
-
Impaired immune responses in streptozotocin-induced type I diabetes in mice. Involvement of high glucose.Clin Exp Immunol. 2008 Nov;154(2):235-46. doi: 10.1111/j.1365-2249.2008.03742.x. Epub 2008 Sep 5. Clin Exp Immunol. 2008. PMID: 18778365 Free PMC article.
-
Expression and immune response to islet antigens following treatment with low doses of streptozotocin in H-2d mice.J Autoimmun. 1997 Feb;10(1):17-25. doi: 10.1006/jaut.1996.0108. J Autoimmun. 1997. PMID: 9080296
-
Essential fatty acid deficiency prevents multiple low-dose streptozotocin-induced diabetes in naive and cyclosporin-treated low-responder murine strains.Acta Diabetol. 1995 Jun;32(2):125-30. doi: 10.1007/BF00569571. Acta Diabetol. 1995. PMID: 7579534
-
Differential immune reactivity to stress in BALB/cByJ and C57BL/6J mice: in vivo dependence on macrophages.Physiol Behav. 1998 Aug;65(1):95-103. doi: 10.1016/s0031-9384(98)00149-8. Physiol Behav. 1998. PMID: 9811371
-
Diabetes Mellitus and Liver Surgery: The Effect of Diabetes on Oxidative Stress and Inflammation.Mediators Inflamm. 2018 May 8;2018:2456579. doi: 10.1155/2018/2456579. eCollection 2018. Mediators Inflamm. 2018. PMID: 29853784 Free PMC article. Review.
Cited by
-
A2BAR Antagonism Decreases the Glomerular Expression and Secretion of Chemoattractants for Monocytes and the Pro-Fibrotic M2 Macrophages Polarization during Diabetic Nephropathy.Int J Mol Sci. 2023 Jun 29;24(13):10829. doi: 10.3390/ijms241310829. Int J Mol Sci. 2023. PMID: 37446007 Free PMC article.
-
The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System.Front Immunol. 2020 Jul 22;11:1582. doi: 10.3389/fimmu.2020.01582. eCollection 2020. Front Immunol. 2020. PMID: 32793223 Free PMC article. Review.
-
Okra [Abelmoschus esculentus (L.) Moench] improved blood glucose and restored histopathological alterations in splenic tissues in a rat model with streptozotocin-induced type 1 diabetes through CD8+ T cells and NF-kβ expression.Front Vet Sci. 2023 Nov 16;10:1268968. doi: 10.3389/fvets.2023.1268968. eCollection 2023. Front Vet Sci. 2023. PMID: 38046568 Free PMC article.
-
The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum.Foods. 2022 Nov 22;11(23):3758. doi: 10.3390/foods11233758. Foods. 2022. PMID: 36496564 Free PMC article.
-
The Effects of Acute Blood Loss for Diagnostic Bloodwork and Fluid Replacement in Clinically Ill Mice.Comp Med. 2015 Jun;65(3):202-16. Comp Med. 2015. PMID: 26141445 Free PMC article.
References
-
- Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. - PubMed
-
- Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. - PubMed
-
- Pozzilli P, Leslie RD. Infections and diabetes: mechanisms and prospects for prevention. Diabet Med. 1994;11:935–941. - PubMed
-
- Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. - PubMed
-
- Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

