Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: model estimates of potential benefits and harms
- PMID: 23381039
- PMCID: PMC3738063
- DOI: 10.7326/0003-4819-158-3-201302050-00003
Comparative effectiveness of alternative prostate-specific antigen--based prostate cancer screening strategies: model estimates of potential benefits and harms
Abstract
Background: The U.S. Preventive Services Task Force recently concluded that the harms of existing prostate-specific antigen (PSA) screening strategies outweigh the benefits.
Objective: To evaluate comparative effectiveness of alternative PSA screening strategies.
Design: Microsimulation model of prostate cancer incidence and mortality quantifying harms and lives saved for alternative PSA screening strategies.
Data sources: National and trial data on PSA growth, screening and biopsy patterns, incidence, treatment distributions, treatment efficacy, and mortality.
Target population: A contemporary cohort of U.S. men.
Time horizon: Lifetime.
Perspective: Societal.
Intervention: 35 screening strategies that vary by start and stop ages, screening intervals, and thresholds for biopsy referral.
Outcome measures: PSA tests, false-positive test results, cancer detected, overdiagnoses, prostate cancer deaths, lives saved, and months of life saved.
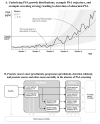
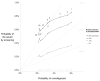
Results of base-case analysis: Without screening, the risk for prostate cancer death is 2.86%. A reference strategy that screens men aged 50 to 74 years annually with a PSA threshold for biopsy referral of 4 µg/L reduces the risk for prostate cancer death to 2.15%, with risk for overdiagnosis of 3.3%. A strategy that uses higher PSA thresholds for biopsy referral in older men achieves a similar risk for prostate cancer death (2.23%) but reduces the risk for overdiagnosis to 2.3%. A strategy that screens biennially with longer screening intervals for men with low PSA levels achieves similar risks for prostate cancer death (2.27%) and overdiagnosis (2.4%), but reduces total tests by 59% and false-positive results by 50%.
Results of sensitivity analysis: Varying incidence inputs or reducing the survival improvement due to screening did not change conclusions.
Limitation: The model is a simplification of the natural history of prostate cancer, and improvement in survival due to screening is uncertain.
Conclusion: Compared with standard screening, PSA screening strategies that use higher thresholds for biopsy referral for older men and that screen men with low PSA levels less frequently can reduce harms while preserving lives.
Primary funding source: National Cancer Institute and Centers for Disease Control and Prevention.
Figures
Comment in
-
Probability, uncertainty, and prostate cancer.Ann Intern Med. 2013 Feb 5;158(3):211-212. doi: 10.7326/0003-4819-158-3-201302050-00012. Ann Intern Med. 2013. PMID: 23381041 No abstract available.
-
Alternative prostate cancer screening strategies.Ann Intern Med. 2013 May 21;158(10):778. doi: 10.7326/0003-4819-158-10-201305210-00019. Ann Intern Med. 2013. PMID: 23689773 No abstract available.
-
Alternative prostate cancer screening strategies--in response.Ann Intern Med. 2013 May 21;158(10):778-9. doi: 10.7326/0003-4819-158-10-201305210-00020. Ann Intern Med. 2013. PMID: 23689774 No abstract available.
-
Words of wisdom: Re: Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: model estimates of potential benefits and harms.Eur Urol. 2013 Oct;64(4):682-3. doi: 10.1016/j.eururo.2013.07.016. Eur Urol. 2013. PMID: 23998500 No abstract available.
Summary for patients in
-
Summaries for patients. Screening smarter, not harder, for prostate cancer.Ann Intern Med. 2013 Feb 5;158(3):I-30. doi: 10.7326/0003-4819-158-3-201302050-00001. Ann Intern Med. 2013. PMID: 23381056 No abstract available.
References
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
