A novel Omega-class glutathione S-transferase gene in Apis cerana cerana: molecular characterisation of GSTO2 and its protective effects in oxidative stress
- PMID: 23382010
- PMCID: PMC3682018
- DOI: 10.1007/s12192-013-0406-2
A novel Omega-class glutathione S-transferase gene in Apis cerana cerana: molecular characterisation of GSTO2 and its protective effects in oxidative stress
Abstract
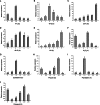
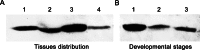
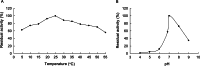
Oxidative stress may be the most significant threat to the survival of living organisms. Glutathione S-transferases (GSTs) serve as the primary defences against xenobiotic and peroxidative-induced oxidative damage. In contrast to other well-defined GST classes, the Omega-class members are poorly understood, particularly in insects. Here, we isolated and characterised the GSTO2 gene from Apis cerana cerana (AccGSTO2). The predicted transcription factor binding sites in the AccGSTO2 promoter suggested possible functions in early development and antioxidant defence. Real-time quantitative PCR (qPCR) and western blot analyses indicated that AccGSTO2 was highly expressed in larvae and was predominantly localised to the brain tissue in adults. Moreover, AccGSTO2 transcription was induced by various abiotic stresses. The purified recombinant AccGSTO2 exhibited glutathione-dependent dehydroascorbate reductase and peroxidase activities. Furthermore, it could prevent DNA damage. In addition, Escherichia coli overexpressing AccGSTO2 displayed resistance to long-term oxidative stress exposure in disc diffusion assays. Taken together, these results suggest that AccGSTO2 plays a protective role in counteracting oxidative stress.
Figures

 ), (inverted filled triangle) and (
), (inverted filled triangle) and ( ), respectively. b Phylogenetic relationships between glutathione transferases (GSTs) from different insect species. The six primary classes are shown, and AccGSTO2 is boxed. c The tertiary structure of AccGSTO2. The conserved G-site residues (Y27, C28, P29, and F30), H-site residues (S115, I118, S119, P173, and E176), N-terminal, and C-terminal are shown
), respectively. b Phylogenetic relationships between glutathione transferases (GSTs) from different insect species. The six primary classes are shown, and AccGSTO2 is boxed. c The tertiary structure of AccGSTO2. The conserved G-site residues (Y27, C28, P29, and F30), H-site residues (S115, I118, S119, P173, and E176), N-terminal, and C-terminal are shown

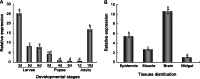


Similar articles
-
Functional and mutational analyses of an omega-class glutathione S-transferase (GSTO2) that is required for reducing oxidative damage in Apis cerana cerana.Insect Mol Biol. 2016 Aug;25(4):470-86. doi: 10.1111/imb.12236. Epub 2016 May 12. Insect Mol Biol. 2016. PMID: 27170478
-
A glutathione S-transferase gene associated with antioxidant properties isolated from Apis cerana cerana.Naturwissenschaften. 2016 Jun;103(5-6):43. doi: 10.1007/s00114-016-1362-3. Epub 2016 Apr 28. Naturwissenschaften. 2016. PMID: 27126403
-
Identification, genomic organization, and oxidative stress response of a sigma class glutathione S-transferase gene (AccGSTS1) in the honey bee, Apis cerana cerana.Cell Stress Chaperones. 2013 Jul;18(4):415-26. doi: 10.1007/s12192-012-0394-7. Epub 2012 Dec 20. Cell Stress Chaperones. 2013. PMID: 23250585 Free PMC article.
-
Identification and characterization of an Apis cerana cerana Delta class glutathione S-transferase gene (AccGSTD) in response to thermal stress.Naturwissenschaften. 2013 Feb;100(2):153-63. doi: 10.1007/s00114-012-1006-1. Epub 2012 Dec 29. Naturwissenschaften. 2013. PMID: 23275971
-
Characterization of the omega class of glutathione transferases.Methods Enzymol. 2005;401:78-99. doi: 10.1016/S0076-6879(05)01005-0. Methods Enzymol. 2005. PMID: 16399380 Review.
Cited by
-
SeGSTo, a novel glutathione S-transferase from the beet armyworm (Spodoptera exigua), involved in detoxification and oxidative stress.Cell Stress Chaperones. 2016 Sep;21(5):805-16. doi: 10.1007/s12192-016-0705-5. Epub 2016 May 26. Cell Stress Chaperones. 2016. PMID: 27230212 Free PMC article.
-
Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance.Mar Drugs. 2019 Mar 1;17(3):147. doi: 10.3390/md17030147. Mar Drugs. 2019. PMID: 30832239 Free PMC article.
-
Biomarkers in glioblastoma and degenerative CNS diseases: defining new advances in clinical usefulness and therapeutic molecular target.Front Mol Biosci. 2025 Mar 18;12:1506961. doi: 10.3389/fmolb.2025.1506961. eCollection 2025. Front Mol Biosci. 2025. PMID: 40171042 Free PMC article.
-
Mechanisms of Pathogen and Pesticide Resistance in Honey Bees.Physiology (Bethesda). 2024 Jul 1;39(4):0. doi: 10.1152/physiol.00033.2023. Epub 2024 Feb 27. Physiology (Bethesda). 2024. PMID: 38411571 Free PMC article. Review.
-
Effect of Sublethal Doses of Imidacloprid on the Biological Performance of Aphid Endoparasitoid Aphidius gifuensis (Hymenoptera: Aphidiidae) and Influence on Its Related Gene Expression.Front Physiol. 2018 Dec 11;9:1729. doi: 10.3389/fphys.2018.01729. eCollection 2018. Front Physiol. 2018. PMID: 30618780 Free PMC article.
References
-
- Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC, Kamath AV, Rosner MH, Chrunyk BA, Perregaux DE, Gabel CA, Geoghegan KF, Pandit J. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J Biol Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

