Changing mechanisms of opiate tolerance and withdrawal during early development: animal models of the human experience
- PMID: 23382147
- PMCID: PMC6040919
- DOI: 10.1093/ilar.52.3.329
Changing mechanisms of opiate tolerance and withdrawal during early development: animal models of the human experience
Abstract
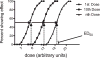
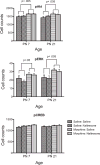
Human infants may be exposed to opiates through placental transfer from an opiate-using mother or through the direct administration of such drugs to relieve pain (e.g., due to illness or neonatal surgery). Infants of many species show physical dependence and tolerance to opiates. The magnitude of tolerance and the nature of withdrawal differ from those of the adult. Moreover, the mechanisms that contribute to the chronic effects of opiates are not well understood in the infant but include biological processes that are both common to and distinct from those of the adult. We review the animal research literature on the effects of chronic and acute opiate exposure in infants and identify mechanisms of withdrawal and tolerance that are similar to and different from those understood in adults. These mechanisms include opioid pharmacology, underlying neural substrates, and the involvement of other neurotransmitter systems. It appears that brain circuitry and opioid receptor types are similar but that NMDA receptor function is immature in the infant. Intracellular signaling cascades may differ but data are complicated by differences between the effects of chronic versus acute morphine treatment. Given the limited treatment options for the dependent infant patient, further study of the biological functions that are altered by chronic opiate treatment is necessary to guide evidenced-based treatment modalities.
Figures





References
-
- Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care. 2009;4:1–5. - PubMed
-
- Bardo MT, Hughes RA. Single-dose tolerance to morphine-induced analgesic and hypoactive effects in infant rats. Dev Psycho biol. 1981;14:415–423. - PubMed
-
- Barr GA, Goodwin GA. Precipitated morphine withdrawal induces a conditioned aversion in the preweaning rat. Pharmacol Biochem Behav. 1997;57:779–783. - PubMed
-
- Barr GA, Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. Eur J Pharmacol. 1992;215:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

