Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions
- PMID: 23382188
- PMCID: PMC3581982
- DOI: 10.1073/pnas.1218020110
Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions
Abstract
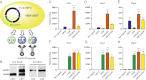
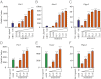
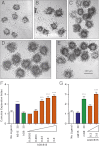
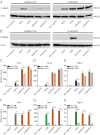
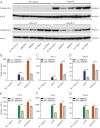
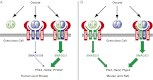
The TGF-β superfamily is the largest family of secreted proteins in mammals, and members of the TGF-β family are involved in most developmental and physiological processes. Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), oocyte-secreted paralogs of the TGF-β superfamily, have been shown genetically to control ovarian physiology. Although previous studies found that GDF9 and BMP15 homodimers can modulate ovarian pathways in vitro, the functional species-specific significance of GDF9:BMP15 heterodimers remained unresolved. Therefore, we engineered and produced purified recombinant mouse and human GDF9 and BMP15 homodimers and GDF9:BMP15 heterodimers to compare their molecular characteristics and physiological functions. In mouse granulosa cell and cumulus cell expansion assays, mouse GDF9 and human BMP15 homodimers can up-regulate cumulus expansion-related genes (Ptx3, Has2, and Ptgs2) and promote cumulus expansion in vitro, whereas mouse BMP15 and human GDF9 homodimers are essentially inactive. However, we discovered that mouse GDF9:BMP15 heterodimer is ∼10- to 30-fold more biopotent than mouse GDF9 homodimer, and human GDF9:BMP15 heterodimer is ∼1,000- to 3,000-fold more bioactive than human BMP15 homodimer. We also demonstrate that the heterodimers require the kinase activities of ALK4/5/7 and BMPR2 to activate SMAD2/3 but unexpectedly need ALK6 as a coreceptor in the signaling complex in granulosa cells. Our findings that GDF9:BMP15 heterodimers are the most bioactive ligands in mice and humans compared with homodimers explain many puzzling genetic and physiological data generated during the last two decades and have important implications for improving female fertility in mammals.
Conflict of interest statement
Conflict of interest statement: Baylor College of Medicine has filed a provisional patent application on the GDF9:BMP15 heterodimer findings; the inventors on this application are M.M.M. and J.P.
Figures

Comment in
-
Growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):E2257. doi: 10.1073/pnas.1303459110. Epub 2013 May 6. Proc Natl Acad Sci U S A. 2013. PMID: 23650403 Free PMC article. No abstract available.
-
Reply to Mottershead et al.: GDF9:BMP15 heterodimers are potent regulators of ovarian functions.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):E2258. doi: 10.1073/pnas.1304497110. Proc Natl Acad Sci U S A. 2013. PMID: 23940843 Free PMC article. No abstract available.
References
-
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian TGF-β superfamily. Endocr Rev. 2002;23:787–823. - PubMed
-
- Matzuk MM, Burns KH. Genetics of mammalian reproduction: Modeling the end of the germline. Annu Rev Physiol. 2012;74:503–528. - PubMed
-
- Mazerbourg S, et al. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol. 2004;18(3):653–665. - PubMed
-
- Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem. 2003;278(1):304–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

