Ikaros promotes early-born neuronal fates in the cerebral cortex
- PMID: 23382203
- PMCID: PMC3581915
- DOI: 10.1073/pnas.1215707110
Ikaros promotes early-born neuronal fates in the cerebral cortex
Erratum in
-
Correction for Alsiö et al., Ikaros promotes early-born neuronal fates in the cerebral cortex.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):E3088. doi: 10.1073/pnas.1508413112. Epub 2015 May 14. Proc Natl Acad Sci U S A. 2015. PMID: 25976099 Free PMC article. No abstract available.
Abstract
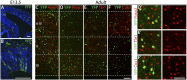
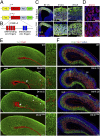
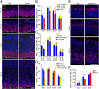
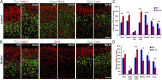
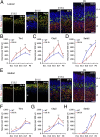
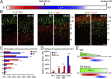
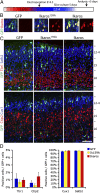
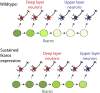
During cerebral cortex development, a series of projection neuron types is generated in a fixed temporal order. In Drosophila neuroblasts, the transcription factor hunchback encodes first-born identity within neural lineages. One of its mammalian homologs, Ikaros, was recently reported to play an equivalent role in retinal progenitor cells, raising the question as to whether Ikaros/Hunchback proteins could be general factors regulating the development of early-born fates throughout the nervous system. Ikaros is also expressed in progenitor cells of the mouse cerebral cortex, and this expression is highest during the early stages of neurogenesis and thereafter decreases over time. Transgenic mice with sustained Ikaros expression in cortical progenitor cells and neurons have developmental defects, including displaced progenitor cells within the cortical plate, increased early neural differentiation, and disrupted cortical lamination. Sustained Ikaros expression results in a prolonged period of generation of deep layer neurons into the stages when, normally, only late-born upper layer neurons are generated, as well as a delayed production of late-born neurons. Consequently, more early-born and fewer late-born neurons are present in the cortex of these mice at birth. This phenotype was observed in all parts of the cortex, including those with minimal structural defects, demonstrating that it is not secondary to abnormalities in cortical morphogenesis. These data suggest that Ikaros plays a similar role in regulating early temporal fates in the mammalian cerebral cortex as Ikaros/Hunchback proteins do in the Drosophila nerve cord.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ikaros confers early temporal competence to mouse retinal progenitor cells.Neuron. 2008 Oct 9;60(1):26-39. doi: 10.1016/j.neuron.2008.08.008. Neuron. 2008. PMID: 18940586
-
The methyl binding domain 3/nucleosome remodelling and deacetylase complex regulates neural cell fate determination and terminal differentiation in the cerebral cortex.Neural Dev. 2015 May 2;10:13. doi: 10.1186/s13064-015-0040-z. Neural Dev. 2015. PMID: 25934499 Free PMC article.
-
Recombineering Hunchback identifies two conserved domains required to maintain neuroblast competence and specify early-born neuronal identity.Development. 2010 May;137(9):1421-30. doi: 10.1242/dev.048678. Epub 2010 Mar 24. Development. 2010. PMID: 20335359 Free PMC article.
-
Postnatal cerebral cortical multipotent progenitors: regulatory mechanisms and potential role in the development of novel neural regenerative strategies.Brain Pathol. 1999 Jul;9(3):515-26. doi: 10.1111/j.1750-3639.1999.tb00539.x. Brain Pathol. 1999. PMID: 10416991 Free PMC article. Review.
-
[Effect of environmental factor influencing the development of mouse cerebral cortex].Yakugaku Zasshi. 2011;131(9):1317-21. doi: 10.1248/yakushi.131.1317. Yakugaku Zasshi. 2011. PMID: 21881305 Review. Japanese.
Cited by
-
How prolonged expression of Hunchback, a temporal transcription factor, re-wires locomotor circuits.Elife. 2019 Sep 10;8:e46089. doi: 10.7554/eLife.46089. Elife. 2019. PMID: 31502540 Free PMC article.
-
Transcriptional and epigenetic regulation of temporal patterning in neural progenitors.Dev Biol. 2022 Jan;481:116-128. doi: 10.1016/j.ydbio.2021.10.006. Epub 2021 Oct 16. Dev Biol. 2022. PMID: 34666024 Free PMC article. Review.
-
Neural specification, targeting, and circuit formation during visual system assembly.Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2101823118. doi: 10.1073/pnas.2101823118. Proc Natl Acad Sci U S A. 2021. PMID: 34183440 Free PMC article.
-
Timing temporal transitions during brain development.Curr Opin Neurobiol. 2017 Feb;42:84-92. doi: 10.1016/j.conb.2016.11.010. Epub 2016 Dec 13. Curr Opin Neurobiol. 2017. PMID: 27984764 Free PMC article. Review.
-
Single cell spatial biology over developmental time can decipher pediatric brain pathologies.Neurobiol Dis. 2024 Sep;199:106597. doi: 10.1016/j.nbd.2024.106597. Epub 2024 Jul 9. Neurobiol Dis. 2024. PMID: 38992777 Review.
References
-
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nat Rev Neurosci. 2001;2(2):109–118. - PubMed
-
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254(5029):282–285. - PubMed
-
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17(1):55–61. - PubMed
-
- Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–221. - PubMed
-
- Alcamo EA, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

