CFTR-deficient pigs display peripheral nervous system defects at birth
- PMID: 23382208
- PMCID: PMC3581923
- DOI: 10.1073/pnas.1222729110
CFTR-deficient pigs display peripheral nervous system defects at birth
Abstract
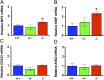
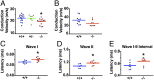
Peripheral nervous system abnormalities, including neuropathy, have been reported in people with cystic fibrosis. These abnormalities have largely been attributed to secondary manifestations of the disease. We tested the hypothesis that disruption of the cystic fibrosis transmembrane conductance regulator (CFTR) gene directly influences nervous system function by studying newborn CFTR(-/-) pigs. We discovered CFTR expression and activity in Schwann cells, and loss of CFTR caused ultrastructural myelin sheath abnormalities similar to those in known neuropathies. Consistent with neuropathic changes, we found increased transcripts for myelin protein zero, a gene that, when mutated, can cause axonal and/or demyelinating neuropathy. In addition, axon density was reduced and conduction velocities of the trigeminal and sciatic nerves were decreased. Moreover, in vivo auditory brainstem evoked potentials revealed delayed conduction of the vestibulocochlear nerve. Our data suggest that loss of CFTR directly alters Schwann cell function and that some nervous system defects in people with cystic fibrosis are likely primary.
Conflict of interest statement
Conflict of interest statement: M.J.W. was a co-founder of Exemplar Genetics, a company to house and produce porcine models of human diseases. M.J.W. holds less than 3% equity and does not receive any money for services.
Figures




Similar articles
-
Cystic Fibrosis and the Nervous System.Chest. 2017 May;151(5):1147-1155. doi: 10.1016/j.chest.2016.11.009. Epub 2016 Nov 19. Chest. 2017. PMID: 27876591 Free PMC article. Review.
-
CFTR-deficient pigs display alterations of bone microarchitecture and composition at birth.J Cyst Fibros. 2020 May;19(3):466-475. doi: 10.1016/j.jcf.2019.10.023. Epub 2019 Nov 29. J Cyst Fibros. 2020. PMID: 31787573
-
Cystic Fibrosis Transmembrane Conductance Regulator in Sarcoplasmic Reticulum of Airway Smooth Muscle. Implications for Airway Contractility.Am J Respir Crit Care Med. 2016 Feb 15;193(4):417-26. doi: 10.1164/rccm.201508-1562OC. Am J Respir Crit Care Med. 2016. PMID: 26488271 Free PMC article.
-
On the mechanism of gating defects caused by the R117H mutation in cystic fibrosis transmembrane conductance regulator.J Physiol. 2016 Jun 15;594(12):3227-44. doi: 10.1113/JP271723. Epub 2016 Mar 23. J Physiol. 2016. PMID: 26846474 Free PMC article.
-
Defects in processing and trafficking of cystic fibrosis transmembrane conductance regulator.Exp Nephrol. 2000 Nov-Dec;8(6):332-42. doi: 10.1159/000020687. Exp Nephrol. 2000. PMID: 11014930 Review.
Cited by
-
Neuronal activity in the hub of extrasynaptic Schwann cell-axon interactions.Front Cell Neurosci. 2013 Nov 25;7:228. doi: 10.3389/fncel.2013.00228. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24324401 Free PMC article.
-
Simple and reproducible approaches for the collection of select porcine ganglia.J Neurosci Methods. 2017 Sep 1;289:93-98. doi: 10.1016/j.jneumeth.2017.06.005. Epub 2017 Jun 8. J Neurosci Methods. 2017. PMID: 28602889 Free PMC article.
-
Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis.Respir Res. 2018 Aug 6;19(1):149. doi: 10.1186/s12931-018-0846-4. Respir Res. 2018. PMID: 30081920 Free PMC article. Review.
-
Identification of cholinergic cells with chemosensory traits in the porcine uterus.Cell Tissue Res. 2022 Apr;388(1):33-47. doi: 10.1007/s00441-022-03585-1. Epub 2022 Jan 27. Cell Tissue Res. 2022. PMID: 35084573 Free PMC article.
-
Monocyte derived macrophages from CF pigs exhibit increased inflammatory responses at birth.J Cyst Fibros. 2017 Jul;16(4):471-474. doi: 10.1016/j.jcf.2017.03.007. Epub 2017 Apr 1. J Cyst Fibros. 2017. PMID: 28377087 Free PMC article.
References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. 2001. Cystic fibrosis. The Metabolic and Molecular Basis of Inherited Disease, eds Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B (McGraw-Hill, New York), 8th Ed, pp 5121–5189.
-
- Quinton PM. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev. 1999;79(1)(Suppl):S3–S22. - PubMed
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. - PubMed
-
- El-Salem K, Aburahma S, Rawashdeh M. Peripheral nerve dysfunction in patients with cystic fibrosis. J Clin Neurophysiol. 2010;27(3):216–218. - PubMed
-
- O’Riordan JI, Hayes J, Fitzgerald MX, Redmond J. Peripheral nerve dysfunction in adult patients with cystic fibrosis. Ir J Med Sci. 1995;164(3):207–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

