ARCH domain of XPD, an anchoring platform for CAK that conditions TFIIH DNA repair and transcription activities
- PMID: 23382212
- PMCID: PMC3581935
- DOI: 10.1073/pnas.1213981110
ARCH domain of XPD, an anchoring platform for CAK that conditions TFIIH DNA repair and transcription activities
Abstract
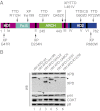
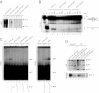
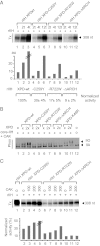
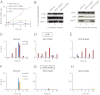
The xeroderma pigmentosum group D (XPD) helicase is a subunit of transcription/DNA repair factor, transcription factor II H (TFIIH) that catalyzes the unwinding of a damaged DNA duplex during nucleotide excision repair. Apart from two canonical helicase domains, XPD is composed of a 4Fe-S cluster domain involved in DNA damage recognition and a module of uncharacterized function termed the "ARCH domain." By investigating the consequences of a mutation found in a patient with trichothiodystrophy, we show that the ARCH domain is critical for the recruitment of the cyclin-dependent kinase (CDK)-activating kinase (CAK) complex. Indeed, this mutation not only affects the interaction with the MAT1 CAK subunit, thereby decreasing the in vitro basal transcription activity of TFIIH itself and impeding the efficient recruitment of the transcription machinery on the promoter of an activated gene, but also impairs the DNA unwinding activity of XPD and the nucleotide excision repair activity of TFIIH. We further demonstrate the role of CAK in downregulating the XPD helicase activity within TFIIH. Taken together, our results identify the ARCH domain of XPD as a platform for the recruitment of CAK and as a potential molecular switch that might control TFIIH composition and play a key role in the conversion of TFIIH from a factor active in transcription to a factor involved in DNA repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- de Boer J, Hoeijmakers JH. Nucleotide excision repair and human syndromes. Carcinogenesis. 2000;21(3):453–460. - PubMed
-
- Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: One gene, two functions, three diseases. Genes Dev. 2001;15(1):15–23. - PubMed
-
- Price VH, Odom RB, Ward WH, Jones FT. Trichothiodystrophy: Sulfur-deficient brittle hair as a marker for a neuroectodermal symptom complex. Arch Dermatol. 1980;116(12):1375–1384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

