Reconstitution of homomeric GluA2(flop) receptors in supported lipid membranes: functional and structural properties
- PMID: 23382380
- PMCID: PMC3605683
- DOI: 10.1074/jbc.M112.422105
Reconstitution of homomeric GluA2(flop) receptors in supported lipid membranes: functional and structural properties
Abstract
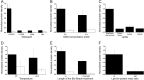
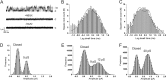
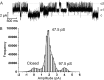
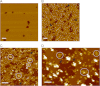
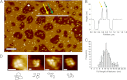
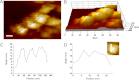
AMPA receptors (AMPARs) are glutamate-gated ion channels ubiquitous in the vertebrate central nervous system, where they mediate fast excitatory neurotransmission and act as molecular determinants of memory formation and learning. Together with detailed analyses of individual AMPAR domains, structural studies of full-length AMPARs by electron microscopy and x-ray crystallography have provided important insights into channel assembly and function. However, the correlation between the structure and functional states of the channel remains ambiguous particularly because these functional states can be assessed only with the receptor bound within an intact lipid bilayer. To provide a basis for investigating AMPAR structure in a membrane environment, we developed an optimized reconstitution protocol using a receptor whose structure has previously been characterized by electron microscopy. Single-channel recordings of reconstituted homomeric GluA2(flop) receptors recapitulate key electrophysiological parameters of the channels expressed in native cellular membranes. Atomic force microscopy studies of the reconstituted samples provide high-resolution images of membrane-embedded full-length AMPARs at densities comparable to those in postsynaptic membranes. The data demonstrate the effect of protein density on conformational flexibility and dimensions of the receptors and provide the first structural characterization of functional membrane-embedded AMPARs, thus laying the foundation for correlated structure-function analyses of the predominant mediators of excitatory synaptic signals in the brain.
Figures


References
-
- Derkach V. A., Oh M. C., Guire E. S., Soderling T. R. (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 8, 101–113 - PubMed
-
- Hollmann M., Hartley M., Heinemann S. (1991) Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252, 851–853 - PubMed
-
- Cull-Candy S., Kelly L., Farrant M. (2006) Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 16, 288–297 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

