Viral population analysis and minority-variant detection using short read next-generation sequencing
- PMID: 23382427
- PMCID: PMC3678329
- DOI: 10.1098/rstb.2012.0205
Viral population analysis and minority-variant detection using short read next-generation sequencing
Abstract
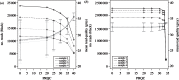
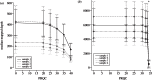
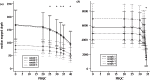
RNA viruses within infected individuals exist as a population of evolutionary-related variants. Owing to evolutionary change affecting the constitution of this population, the frequency and/or occurrence of individual viral variants can show marked or subtle fluctuations. Since the development of massively parallel sequencing platforms, such viral populations can now be investigated to unprecedented resolution. A critical problem with such analyses is the presence of sequencing-related errors that obscure the identification of true biological variants present at low frequency. Here, we report the development and assessment of the Quality Assessment of Short Read (QUASR) Pipeline (http://sourceforge.net/projects/quasr) specific for virus genome short read analysis that minimizes sequencing errors from multiple deep-sequencing platforms, and enables post-mapping analysis of the minority variants within the viral population. QUASR significantly reduces the error-related noise in deep-sequencing datasets, resulting in increased mapping accuracy and reduction of erroneous mutations. Using QUASR, we have determined influenza virus genome dynamics in sequential samples from an in vitro evolution of 2009 pandemic H1N1 (A/H1N1/09) influenza from samples sequenced on both the Roche 454 GSFLX and Illumina GAIIx platforms. Importantly, concordance between the 454 and Illumina sequencing allowed unambiguous minority-variant detection and accurate determination of virus population turnover in vitro.
Figures


References
-
- Bunnik EM, et al. 2011. Detection of inferred CCR5- and CXCR4-using HIV-1 variants and evolutionary intermediates using ultra-deep pyrosequencing. PLoS Pathog. 7, e1002106 (doi:10.1371/journal.ppat.1002106) - DOI - PMC - PubMed
-
- Moya A, Holmes E, González-Candelas F. 2004. The population genetics and evolutionary epidemiology of RNA viruses. Nat. Rev. Microbiol. 2, 279–288 (doi:10.1038/nrmicro863) - DOI - PMC - PubMed
-
- Wang C, Mitsuya Y, Gharizadeh B, Ronaghi M, Shafer RW. 2007. Characterization of mutation spectra with ultra-deep pyrosequencing: application to HIV-1 drug resistance. Genome Res. 17, 1195–1201 (doi:10.1101/gr.6468307) - DOI - PMC - PubMed
-
- Archer J, Braverman MS, Taillon BE, Desany B, James I, Harrigan PR, Lewis M, Robertson DL. 2009. Detection of low-frequency pretherapy chemokine (CXC motif) receptor 4 (CXCR4)-using HIV-1 with ultra-deep pyrosequencing. AIDS 23, 1209–1218 (doi:10.1097/QAD.0b013e32832b4399) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

