The tomato Prf complex is a molecular trap for bacterial effectors based on Pto transphosphorylation
- PMID: 23382672
- PMCID: PMC3561153
- DOI: 10.1371/journal.ppat.1003123
The tomato Prf complex is a molecular trap for bacterial effectors based on Pto transphosphorylation
Abstract
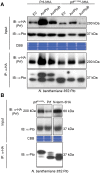
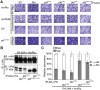
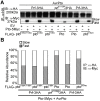
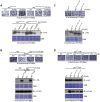
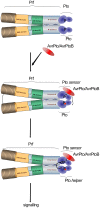
The major virulence strategy of phytopathogenic bacteria is to secrete effector proteins into the host cell to target the immune machinery. AvrPto and AvrPtoB are two such effectors from Pseudomonas syringae, which disable an overlapping range of kinases in Arabidopsis and Tomato. Both effectors target surface-localized receptor-kinases to avoid bacterial recognition. In turn, tomato has evolved an intracellular effector-recognition complex composed of the NB-LRR protein Prf and the Pto kinase. Structural analyses have shown that the most important interaction surface for AvrPto and AvrPtoB is the Pto P+1 loop. AvrPto is an inhibitor of Pto kinase activity, but paradoxically, this kinase activity is a prerequisite for defense activation by AvrPto. Here using biochemical approaches we show that disruption of Pto P+1 loop stimulates phosphorylation in trans, which is possible because the Pto/Prf complex is oligomeric. Both P+1 loop disruption and transphosphorylation are necessary for signalling. Thus, effector perturbation of one kinase molecule in the complex activates another. Hence, the Pto/Prf complex is a sophisticated molecular trap for effectors that target protein kinases, an essential aspect of the pathogen's virulence strategy. The data presented here give a clear view of why bacterial virulence and host recognition mechanisms are so often related and how the slowly evolving host is able to keep pace with the faster-evolving pathogen.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11: 539–548. - PubMed
-
- Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406. - PubMed
-
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

