Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization
- PMID: 23382677
- PMCID: PMC3561285
- DOI: 10.1371/journal.ppat.1003138
Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization
Abstract
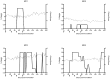
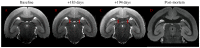
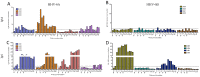
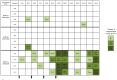
Human Herpesvirus 6 (HHV-6) is a ubiquitous virus with an estimated seroprevalence of 95% in the adult population. HHV-6 is associated with several neurologic disorders, including multiple sclerosis, an inflammatory demyelinating disease affecting the CNS. Animal models of HHV-6 infection would help clarify its role in human disease but have been slow to develop because rodents lack CD46, the receptor for cellular entry. Therefore, we investigated the effects of HHV-6 infections in a non-human primate, the common marmoset Callithrix jacchus. We inoculated a total of 12 marmosets with HHV-6A and HHV-6B intravenously and HHV-6A intranasally. Animals were monitored for 25 weeks post-inoculation clinically, immunologically and by MRI. Marmosets inoculated with HHV-6A intravenously exhibited neurologic symptoms and generated virus-specific antibody responses, while those inoculated intravenously with HHV-6B were asymptomatic and generated comparatively lower antibody responses. Viral DNA was detected at a low frequency in paraffin-embedded CNS tissue of a subset of marmosets inoculated with HHV-6A and HHV-6B intravenously. When different routes of HHV-6A inoculation were compared, intravenous inoculation resulted in virus-specific antibody responses and infrequent detection of viral DNA in the periphery, while intranasal inoculation resulted in negligible virus-specific antibody responses and frequent detection of viral DNA in the periphery. Moreover, marmosets inoculated with HHV-6A intravenously exhibited neurologic symptoms, while marmosets inoculated with HHV-6A intranasally were asymptomatic. We demonstrate that a marmoset model of HHV-6 infection can serve to further define the contribution of this ubiquitous virus to human neurologic disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Pellett PE, Dominguez G. (2001) Chapter 80. Human Herpesviruses 6A, 6B, and 7 and Their Replication. In: Knipe DM, Howley, P.M., editor. Fields Virology. 4th edition. Philadelphia: Lippincott, Williams & Wilkins. pp. 69–2784.
-
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, et al. (1986) Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234: 596–601. - PubMed
-
- De Bolle L, Van Loon J, De Clercq E, Naesens L (2005) Quantitative analysis of human herpesvirus 6 cell tropism. J Med Virol 75: 76–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

