Lipid- and polymer-based nanostructures for cancer theranostics
- PMID: 23382770
- PMCID: PMC3563151
- DOI: 10.7150/thno.4381
Lipid- and polymer-based nanostructures for cancer theranostics
Abstract
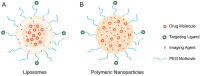
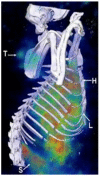
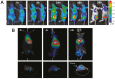
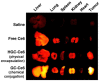
The relatively new field of nanotheranostics combines the advantages of in vivo diagnosis with the ability to administer treatment through a single nano-sized carrier, offering new opportunities for cancer diagnosis and therapy. Nanotheranostics has facilitated the development of nanomedicine through direct visualization of drug blood circulation and biodistribution. From a clinical perspective, nanotheranostics allows therapies to be administered and monitored in real time, thus decreasing the potential of under- or over-dosing and allowing for more personalized treatment regimens. Herein, we review recent development of nanotheranostics using lipid- and polymer-based formulations, with a particular focus on their applications in cancer research. Recent advances in nanotechnology aimed to combine therapeutic molecules with imaging agents for magnetic resonance imaging, radionuclide imaging, or fluorescence imaging are discussed.
Keywords: fluorescence imaging; liposomes; magnetic resonance imaging; nanotheranostics; polymeric nanoparticles; radionuclide imaging.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Morgan B. Opportunities and pitfalls of cancer imaging in clinical trials. Nat Rev Clin Oncol. 2011;8:517–27. - PubMed
-
- Sumer B, Gao JM. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3:137–40. - PubMed
-
- Sun D. Nanotheranostics: Integration of imaging and targeted drug delivery. Mol Pharm. 2010;7:1879. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

