Optimization of the ultrasound-induced blood-brain barrier opening
- PMID: 23382778
- PMCID: PMC3563154
- DOI: 10.7150/thno.5576
Optimization of the ultrasound-induced blood-brain barrier opening
Abstract
Current treatments of neurological and neurodegenerative diseases are limited due to the lack of a truly non-invasive, transient, and regionally selective brain drug delivery method. The brain is particularly difficult to deliver drugs to because of the blood-brain barrier (BBB). The impermeability of the BBB is due to the tight junctions connecting adjacent endothelial cells and highly regulatory transport systems of the endothelial cell membranes. The main function of the BBB is ion and volume regulation to ensure conditions necessary for proper synaptic and axonal signaling. However, the same permeability properties that keep the brain healthy also constitute the cause of the tremendous obstacles posed in its pharmacological treatment. The BBB prevents most neurologically active drugs from entering the brain and, as a result, has been isolated as the rate-limiting factor in brain drug delivery. Until a solution to the trans-BBB delivery problem is found, treatments of neurological diseases will remain impeded. Over the past decade, methods that combine Focused Ultrasound (FUS) and microbubbles have been shown to offer the unique capability of noninvasively, locally and transiently open the BBB so as to treat central nervous system (CNS) diseases. Four of the main challenges that have been taken on by our group and discussed in this paper are: 1) assess its safety profile, 2) unveil the mechanism by which the BBB opens and closes, 3) control and predict the opened BBB properties and duration of the opening and 4) assess its premise in brain drug delivery. All these challenges will be discussed, findings in both small (mice) and large (non-human primates) animals are shown and finally the clinical potential for this technique is shown.
Keywords: blood-brain barrier; drug delivery; focused ultrasound; mechanism; microbubble..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


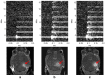
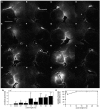
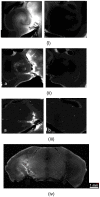

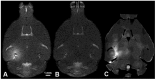

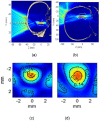
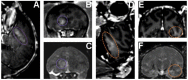
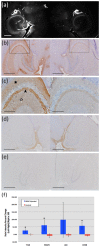
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Stewart P Aand Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function. J Comp Neurol. 1994;340:566–76. - PubMed
-
- Pardridge WM. Molecular trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6(5):494–500. - PubMed
-
- Bakay L, Ballantine HT Jr, Hueter TF. et al. Ultrasonically produced changes in the blood-brain barrier. AMA Arch Neurol Psychiatry. 1956;76:457–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

