The mechanism of effective electroacupuncture on T cell response in rats with experimental autoimmune encephalomyelitis
- PMID: 23382807
- PMCID: PMC3557272
- DOI: 10.1371/journal.pone.0051573
The mechanism of effective electroacupuncture on T cell response in rats with experimental autoimmune encephalomyelitis
Abstract
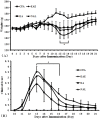
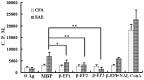
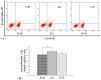
Previously, we demonstrated that electroacupuncture (EA) decreased lymphocyte infiltration into the spinal cords of rats presenting with experimental autoimmune encephalomyelitis (EAE), a disease model used in the study of multiple sclerosis (MS). The aim of this study was to characterize the effects of EA on the EAE. Female Lewis rats were divided into either CFA, EAE, EA, or injection with naloxone after electroacupuncture (NAL) groups. Electroacupuncture was administered every day for 21 days. To evaluate proliferation and apoptosis, lymphocytes from rats presenting with EAE were collected and cultured with β-endorphin. Immunohistochemisty, flow cytometry and radio-immunity methods were applied to detect the expression of β-endorphin. Results presented in this report demonstrate that the beneficial anti-inflammatory effects of EA on EAE were related to β-endorphin production that balances the Thl/Th2 and Th17/Treg responses. These results suggest that β-endorphin could be an important component in the development of EA-based therapies used for the treatment of EAE.
Conflict of interest statement
Figures






References
-
- Gironi M, Martinelli V, Brambilla E, Furlan R, Panerai AE, et al. (2000) Beta-endorphin concentrations in peripheral blood mononuclear cells of patients with multiple sclerosis: effects of treatment with interferon beta. Arch Neurol 57: 1178–1181. - PubMed
-
- Yoshino S, Koiwa M, Shiga H, Nakamura H, Higaki M, et al. (1992) Miyasaka N. Detection of opioid peptides in synovial tissues of patients with rheumatoid arthritis. J Rhematol 19: 660–661. - PubMed
-
- Börner C, Warnick B, Smida M, Hartig R, Lindquist JA, et al. (2009) Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 183(2): 882–889. - PubMed
-
- Panerai AE, Sacerdote P (1997) Beta-endorphin in the immune system: a role at last? Immunol Today 18: 317–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

