40S ribosome biogenesis co-factors are essential for gametophyte and embryo development
- PMID: 23382868
- PMCID: PMC3559688
- DOI: 10.1371/journal.pone.0054084
40S ribosome biogenesis co-factors are essential for gametophyte and embryo development
Abstract
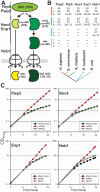
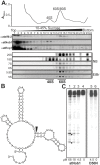
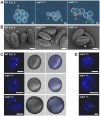
Ribosome biogenesis is well described in Saccharomyces cerevisiae. In contrast only very little information is available on this pathway in plants. This study presents the characterization of five putative protein co-factors of ribosome biogenesis in Arabidopsis thaliana, namely Rrp5, Pwp2, Nob1, Enp1 and Noc4. The characterization of the proteins in respect to localization, enzymatic activity and association with pre-ribosomal complexes is shown. Additionally, analyses of T-DNA insertion mutants aimed to reveal an involvement of the plant co-factors in ribosome biogenesis. The investigated proteins localize mainly to the nucleolus or the nucleus, and atEnp1 and atNob1 co-migrate with 40S pre-ribosomal complexes. The analysis of T-DNA insertion lines revealed that all proteins are essential in Arabidopsis thaliana and mutant plants show alterations of rRNA intermediate abundance already in the heterozygous state. The most significant alteration was observed in the NOB1 T-DNA insertion line where the P-A3 fragment, a 23S-like rRNA precursor, accumulated. The transmission of the T-DNA through the male and female gametophyte was strongly inhibited indicating a high importance of ribosome co-factor genes in the haploid stages of plant development. Additionally impaired embryogenesis was observed in some mutant plant lines. All results support an involvement of the analyzed proteins in ribosome biogenesis but differences in rRNA processing, gametophyte and embryo development suggested an alternative regulation in plants.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

