Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients
- PMID: 23382909
- PMCID: PMC3559838
- DOI: 10.1371/journal.pone.0054522
Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/df796504-a2ea-4807-a412-85985bb2550b
Abstract
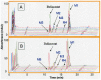
Belinostat is a hydroxamate class HDAC inhibitor that has demonstrated activity in peripheral T-cell lymphoma and is undergoing clinical trials for non-hematologic malignancies. We studied the pharmacokinetics of belinostat in hepatocellular carcinoma patients to determine the main pathway of metabolism of belinostat. The pharmacokinetics of belinostat in liver cancer patients were characterized by rapid plasma clearance of belinostat with extensive metabolism with more than 4-fold greater relative systemic exposure of major metabolite, belinostat glucuronide than that of belinostat. There was significant interindividual variability of belinostat glucuronidation. The major pathway of metabolism involves UGT1A1-mediated glucuronidation and a good correlation has been identified between belinostat glucuronide formation and glucuronidation of known UGT1A1 substrates. In addition, liver microsomes harboring UGT1A1*28 alleles have lower glucuronidation activity for belinostat compared to those with wildtype UGT1A1. The main metabolic pathway of belinostat is through glucuronidation mediated primarily by UGT1A1, a highly polymorphic enzyme. The clinical significance of this finding remains to be determined.
Trial registration: ClinicalTrials.gov NCT00321594.
Conflict of interest statement
Figures






References
-
- Marks PA, Richon VM, Miller T, Kelly WK (2004) Histone deacetylase inhibitors. Adv Cancer Res 91: 137–168. - PubMed
-
- Gray SG, Qian CN, Furge K, Guo X, The BT (2004) Microarray profiling of the effects of histone deacetylase inhibitors on gene expression in cancer cell lines. Int J Oncol 24: 773–795. - PubMed
-
- Tanji N, Ozawa A, Kikugawa T, Miura N, Sasaki T, et al. (2011) Potential of histone deacetylase inhibitors for bladder cancer treatment. Expert Rev Anticancer Ther 1: 959–965. - PubMed

