Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea
- PMID: 23382975
- PMCID: PMC3558510
- DOI: 10.1371/journal.pone.0054807
Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea
Abstract
Background: Obstructive sleep apnea (OSA) is characterized by recurrent nocturnal hypoxia and sleep disruption. Sleep fragmentation caused hyperalgesia in volunteers, while nocturnal hypoxemia enhanced morphine analgesic potency in children with OSA. This evidence directly relates to surgical OSA patients who are at risk for airway compromise due to postoperative use of opioids. Using accepted experimental pain models, we characterized pain processing and opioid analgesia in male volunteers recruited based on their risk for OSA.
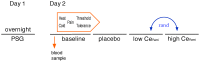
Methods: After approval from the Intitutional Review Board and informed consent, we assessed heat and cold pain thresholds and tolerances in volunteers after overnight polysomnography (PSG). Three pro-inflammatory and 3 hypoxia markers were determined in the serum. Pain tests were performed at baseline, placebo, and two effect site concentrations of remifentanil (1 and 2 µg/ml), an μ-opioid agonist. Linear mixed effects regression models were employed to evaluate the association of 3 PSG descriptors [wake after sleep onset, number of sleep stage shifts, and lowest oxyhemoglobin saturation (SaO(2)) during sleep] and all serum markers with pain thresholds and tolerances at baseline, as well as their changes under remifentanil.
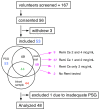
Results: Forty-three volunteers (12 normal and 31 with a PSG-based diagnosis of OSA) were included in the analysis. The lower nadir SaO(2) and higher insulin growth factor binding protein-1 (IGFBP-1) were associated with higher analgesic sensitivity to remifentanil (SaO(2), P = 0.0440; IGFBP-1, P = 0.0013). Other pro-inflammatory mediators like interleukin-1β and tumor necrosis factor-α (TNF-α) were associated with an enhanced sensitivity to the opioid analgesic effect (IL-1β, P = 0.0218; TNF-α, P = 0.0276).
Conclusions: Nocturnal hypoxemia in subjects at high risk for OSA was associated with an increased potency of opioid analgesia. A serum hypoxia marker (IGFBP-1) was associated with hypoalgesia and increased potency to opioid analgesia; other pro-inflammatory mediators also predicted an enhanced opioid potency.
Trial registration: Clinicaltrials.gov NCT00672737.
Conflict of interest statement
Figures
References
-
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, et al. (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235. - PubMed
-
- Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239. - PubMed
-
- Finkel KJ, Searleman AC, Tymkew H, Tanaka CY, Saager L, et al. (2009) Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 10: 753–758. - PubMed
-
- Young T, Evans L, Finn L, Palta M (1997) Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20: 705–706. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

