Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons
- PMID: 23382999
- PMCID: PMC3561411
- DOI: 10.1371/journal.pone.0054914
Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons
Abstract
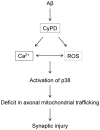
Normal axonal mitochondrial transport and function is essential for the maintenance of synaptic function. Abnormal mitochondrial motility and mitochondrial dysfunction within axons are critical for amyloid β (Aβ)-induced synaptic stress and the loss of synapses relevant to the pathogenesis of Alzheimer's disease (AD). However, the mechanisms controlling axonal mitochondrial function and transport alterations in AD remain elusive. Here, we report an unexplored role of cyclophilin D (CypD)-dependent mitochondrial permeability transition pore (mPTP) in Aβ-impaired axonal mitochondrial trafficking. Depletion of CypD significantly protects axonal mitochondrial motility and dynamics from Aβ toxicity as shown by increased axonal mitochondrial density and distribution and improved bidirectional transport of axonal mitochondria. Notably, blockade of mPTP by genetic deletion of CypD suppresses Aβ-mediated activation of the p38 mitogen-activated protein kinase signaling pathway, reverses axonal mitochondrial abnormalities, improves synaptic function, and attenuates loss of synapse, suggesting a role of CypD-dependent signaling in Aβ-induced alterations in axonal mitochondrial trafficking. The potential mechanisms of the protective effects of lacking CypD on Aβ-induced abnormal mitochondrial transport in axon are increased axonal calcium buffer capability, diminished reactive oxygen species (ROS), and suppressing downstream signal transduction P38 activation. These findings provide new insights into CypD-dependent mitochondrial mPTP and signaling on mitochondrial trafficking in axons and synaptic degeneration in an environment enriched for Aβ.
Conflict of interest statement
Figures






References
-
- Miller KE, Sheetz MP (2004) Axonal mitochondrial transport and potential are correlated. Journal of cell science 117: 2791–2804. - PubMed
-
- Talbot JD, David G, Barrett EF (2003) Inhibition of mitochondrial Ca2+ uptake affects phasic release from motor terminals differently depending on external [Ca2+]. Journal of neurophysiology 90: 491–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

