A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea
- PMID: 23383016
- PMCID: PMC3557228
- DOI: 10.1371/journal.pone.0054966
A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea
Abstract
Defensins are small cationic peptides that could be used as the potential substitute for antibiotics. However, there is no efficient method for producing defensins. In this study, we developed a new strategy to produce defensin in nitrate reductase (NR)-deficient C. ellipsoidea (nrm-4). We constructed a plant expression vector carrying mutated NP-1 gene (mNP-1), a mature α-defensin NP-1 gene from rabbit with an additional initiator codon in the 5'-terminus, in which the selection markers were NptII and NR genes. We transferred mNP-1 into nrm-4 using electroporation and obtained many transgenic lines with high efficiency under selection chemicals G418 and NaNO(3). The mNP-1 was characterized using N-terminal sequencing after being isolated from transgenic lines. Excitingly, mNP-1 was produced at high levels (approximately 11.42 mg/l) even after 15 generations of continuous fermentation. In addition, mNP-1 had strong activity against Escherichia coli at 5 µg/ml. This research developed a new method for producing defensins using genetic engineering.
Conflict of interest statement
Figures
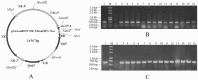
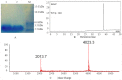

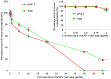
References
-
- Ganz T (2003) The role of antimicrobial peptides in innate immunity. Integr Comp Biol 43: 300–304. - PubMed
-
- Ganz T (2004) Defensins: antimicrobial peptides of vertebrates. Cr Biol 327: 539–549. - PubMed
-
- Giuliani A, Pirri G, Nicoletto SF (2007) Antimicrobial peptides: an overview of a promising class of therapeutics. CEJB 2: 1–33.
-
- Hancock REW, Sah HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24: 1551–1557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

