Dengue virus enhances thrombomodulin and ICAM-1 expression through the macrophage migration inhibitory factor induction of the MAPK and PI3K signaling pathways
- PMID: 23383040
- PMCID: PMC3557271
- DOI: 10.1371/journal.pone.0055018
Dengue virus enhances thrombomodulin and ICAM-1 expression through the macrophage migration inhibitory factor induction of the MAPK and PI3K signaling pathways
Abstract
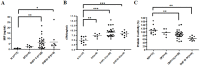
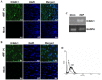
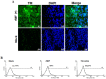
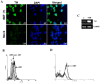
Dengue virus (DV) infections cause mild dengue fever (DF) or severe life-threatening dengue hemorrhagic fever (DHF). The mechanisms that cause hemorrhage in DV infections remain poorly understood. Thrombomodulin (TM) is a glycoprotein expressed on the surface of vascular endothelial cells that plays an important role in the thrombin-mediated activation of protein C. Prior studies have shown that the serum levels of soluble TM (sTM) and macrophage migration inhibitory factor (MIF) are significantly increased in DHF patients compared to levels in DF patients or normal controls. In this study, we investigated how MIF and sTM concentrations are enhanced in the plasma of DHF patients and the potential effect of MIF on coagulation through its influence on two factors: thrombomodulin (TM) and intercellular adhesion molecule-1 (ICAM-1) in endothelial cells and monocytes. Recombinant human macrophage migration inhibitory factor (rMIF) was used to treat monocytic THP-1 cells and endothelial HMEC-1 cells or primary HUVEC cells. The subsequent expression of TM and ICAM-1 was assessed by immunofluorescent staining and flow cytometry analysis. Additionally, the co-incubation of THP-1 cells with various cell signaling pathway inhibitors was used to determine the pathways through which MIF mediated its effect. The data provided evidence that severe DV infections induce MIF expression, which in turn stimulates monocytes or endothelial cells to express TM and ICAM-1 via the Erk, JNK MAPK and the PI3K signaling pathways, supporting the idea that MIF may play an important role as a regulator of coagulation.
Conflict of interest statement
Figures



References
-
- Mairuhu AT, Mac Gillavry MR, Setiati TE, Soemantri A, ten Cate H, et al. (2003) Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis 3: 33–41. - PubMed
-
- Huang YH, Liu CC, Wang ST, Lei HY, Liu HL, et al. (2001) Activation of coagulation and fibrinolysis during dengue virus infection. J Med Virol 63: 247–251. - PubMed
-
- van Gorp EC, Minnema MC, Suharti C, Mairuhu AT, Brandjes DP, et al. (2001) Activation of coagulation factor XI, without detectable contact activation in dengue haemorrhagic fever. Br J Haematol 113: 94–99. - PubMed
-
- Suharti C, van Gorp EC, Setiati TE, Dolmans WM, Djokomoeljanto RJ, et al. (2002) The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb Haemost 87: 42–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

